Loss of CDKL5 impairs survival and dendritic growth of newborn neurons by altering AKT/GSK-3β signaling
- PMID: 24952363
- PMCID: PMC4146476
- DOI: 10.1016/j.nbd.2014.06.006
Loss of CDKL5 impairs survival and dendritic growth of newborn neurons by altering AKT/GSK-3β signaling
Abstract
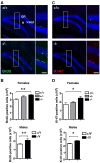
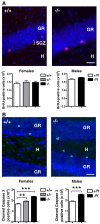
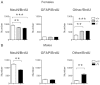
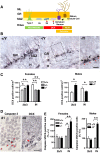
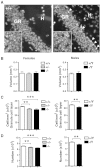
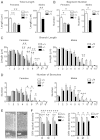
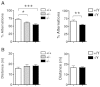
Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5) gene have been identified in a neurodevelopmental disorder characterized by early-onset intractable seizures, severe developmental delay, intellectual disability, and Rett's syndrome-like features. Since the physiological functions of CDKL5 still need to be elucidated, in the current study we took advantage of a new Cdkl5 knockout (KO) mouse model in order to shed light on the role of this gene in brain development. We mainly focused on the hippocampal dentate gyrus, a region that largely develops postnatally and plays a key role in learning and memory. Looking at the process of neurogenesis, we found a higher proliferation rate of neural precursors in Cdkl5 KO mice in comparison with wild type mice. However, there was an increase in apoptotic cell death of postmitotic granule neuron precursors, with a reduction in total number of granule cells. Looking at dendritic development, we found that in Cdkl5 KO mice the newly-generated granule cells exhibited a severe dendritic hypotrophy. In parallel, these neurodevelopmental defects were associated with impairment of hippocampus-dependent memory. Looking at the mechanisms whereby CDKL5 exerts its functions, we identified a central role of the AKT/GSK-3β signaling pathway. Overall our findings highlight a critical role of CDKL5 in the fundamental processes of brain development, namely neuronal precursor proliferation, survival and maturation. This evidence lays the basis for a better understanding of the neurological phenotype in patients carrying mutations in the CDKL5 gene.
Keywords: AKT/GSK-3β signaling; CDKL5 disorder; Dendritic development; Neurodevelopmental disorders; Neurogenesis impairment; Rett's syndrome.
Copyright © 2014. Published by Elsevier Inc.
Figures



References
-
- Armstrong D.D., Dunn J.K., Schultz R.J., Herbert D.A., Glaze D.G., Motil K.J. Organ growth in Rett syndrome: a postmortem examination analysis. Pediatr. Neurol. 1999;20:125–129. - PubMed
-
- Bahi-Buisson N., Kaminska A., Boddaert N., Rio M., Afenjar A., Gerard M., Giuliano F., Motte J., Heron D., Morel M.A., Plouin P., Richelme C., des Portes V., Dulac O., Philippe C., Chiron C., Nabbout R., Bienvenu T. The three stages of epilepsy in patients with CDKL5 mutations. Epilepsia. 2008;49:1027–1037. - PubMed
-
- Bahi-Buisson N., Nectoux J., Rosas-Vargas H., Milh M., Boddaert N., Girard B., Cances C., Ville D., Afenjar A., Rio M., Heron D., N'Guyen Morel M.A., Arzimanoglou A., Philippe C., Jonveaux P., Chelly J., Bienvenu T. Key clinical features to identify girls with CDKL5 mutations. Brain. 2008;131:2647–2661. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

