Nervous translation, do you get the message? A review of mRNPs, mRNA-protein interactions and translational control within cells of the nervous system
- PMID: 24952431
- PMCID: PMC11113408
- DOI: 10.1007/s00018-014-1660-x
Nervous translation, do you get the message? A review of mRNPs, mRNA-protein interactions and translational control within cells of the nervous system
Abstract
In neurons, translation of a message RNA can occur metres away from its transcriptional origin and in normal cells this is orchestrated with perfection. The life of an mRNA will see it pass through multiple steps of processing in the nucleus and the cytoplasm before it reaches its final destination. Processing of mRNA is determined by a myriad of RNA-binding proteins in multi-protein complexes called messenger ribonucleoproteins; however, incorrect processing and delivery of mRNA can cause several human neurological disorders. This review takes us through the life of mRNA from the nucleus to its point of translation in the cytoplasm. The review looks at the various cis and trans factors that act on the mRNA and discusses their roles in different cells of the nervous system and human disorders.
Figures
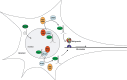
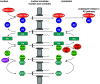
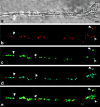
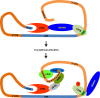

Similar articles
-
Synergistic stimulation of HIV-1 rev-dependent export of unspliced mRNA to the cytoplasm by hnRNP A1.J Mol Biol. 1999 Feb 5;285(5):1951-64. doi: 10.1006/jmbi.1998.2473. J Mol Biol. 1999. PMID: 9925777
-
How cells get the message: dynamic assembly and function of mRNA-protein complexes.Nat Rev Genet. 2013 Apr;14(4):275-87. doi: 10.1038/nrg3434. Epub 2013 Mar 12. Nat Rev Genet. 2013. PMID: 23478349 Review.
-
Extensive association of HuR with hnRNP proteins within immunoselected hnRNP and mRNP complexes.Biochim Biophys Acta. 2010 Apr;1804(4):692-703. doi: 10.1016/j.bbapap.2009.11.007. Epub 2009 Nov 18. Biochim Biophys Acta. 2010. PMID: 19931428
-
mRNA trafficking and local translation: the Yin and Yang of regulating mRNA localization in neurons.Acta Biochim Biophys Sin (Shanghai). 2011 Sep;43(9):663-70. doi: 10.1093/abbs/gmr058. Epub 2011 Jul 11. Acta Biochim Biophys Sin (Shanghai). 2011. PMID: 21749992 Review.
-
The nuclear nurture and cytoplasmic nature of localized mRNPs.Semin Cell Dev Biol. 2007 Apr;18(2):186-93. doi: 10.1016/j.semcdb.2007.01.002. Epub 2007 Jan 23. Semin Cell Dev Biol. 2007. PMID: 17459736 Review.
Cited by
-
Linking RNA Dysfunction and Neurodegeneration in Amyotrophic Lateral Sclerosis.Neurotherapeutics. 2015 Apr;12(2):340-51. doi: 10.1007/s13311-015-0340-3. Neurotherapeutics. 2015. PMID: 25689976 Free PMC article. Review.
-
Eukaryotic elongation factor 2 kinase regulates the synthesis of microtubule-related proteins in neurons.J Neurochem. 2016 Jan;136(2):276-84. doi: 10.1111/jnc.13407. Epub 2015 Nov 17. J Neurochem. 2016. PMID: 26485687 Free PMC article.
-
Exosomal Protein Deficiencies: How Abnormal RNA Metabolism Results in Childhood-Onset Neurological Diseases.J Neuromuscul Dis. 2015;2(Suppl 2):S31-S37. doi: 10.3233/JND-150086. Epub 2015 Jul 22. J Neuromuscul Dis. 2015. PMID: 27127732 Free PMC article.
-
The hnRNP RALY regulates PRMT1 expression and interacts with the ALS-linked protein FUS: implication for reciprocal cellular localization.Mol Biol Cell. 2018 Dec 15;29(26):3067-3081. doi: 10.1091/mbc.E18-02-0108. Epub 2018 Oct 24. Mol Biol Cell. 2018. PMID: 30354839 Free PMC article.
-
Accumulation of poly(A) RNA in nuclear granules enriched in Sam68 in motor neurons from the SMNΔ7 mouse model of SMA.Sci Rep. 2018 Jun 25;8(1):9646. doi: 10.1038/s41598-018-27821-3. Sci Rep. 2018. PMID: 29941967 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

