XIAP inhibitors induce differentiation and impair clonogenic capacity of acute myeloid leukemia stem cells
- PMID: 24952669
- PMCID: PMC4147327
- DOI: 10.18632/oncotarget.2016
XIAP inhibitors induce differentiation and impair clonogenic capacity of acute myeloid leukemia stem cells
Abstract
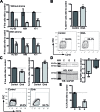
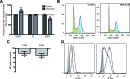
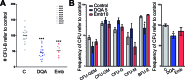
Acute myeloid leukemia (AML) is a neoplasia characterized by the rapid expansion of immature myeloid blasts in the bone marrow, and marked by poor prognosis and frequent relapse. As such, new therapeutic approaches are required for remission induction and prevention of relapse. Due to the higher chemotherapy sensitivity and limited life span of more differentiated AML blasts, differentiation-based therapies are a promising therapeutic approach. Based on public available gene expression profiles, a myeloid-specific differentiation-associated gene expression pattern was defined as the therapeutic target. A XIAP inhibitor (Dequalinium chloride, DQA) was identified in an in silico screening searching for small molecules that induce similar gene expression regulation. Treatment with DQA, similarly to Embelin (another XIAP inhibitor), induced cytotoxicity and differentiation in AML. XIAP inhibition differentially impaired cell viability of the most primitive AML blasts and reduced clonogenic capacity of AML cells, sparing healthy mature blood and hematopoietic stem cells. Taken together, these results suggest that XIAP constitutes a potential target for AML treatment and support the evaluation of XIAP inhibitors in clinical trials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Combined inhibition of XIAP and autophagy induces apoptosis and differentiation in acute myeloid leukaemia.J Cell Mol Med. 2023 Jun;27(12):1682-1696. doi: 10.1111/jcmm.17765. Epub 2023 May 8. J Cell Mol Med. 2023. PMID: 37154878 Free PMC article.
-
Mechanism and functional role of XIAP and Mcl-1 down-regulation in flavopiridol/vorinostat antileukemic interactions.Mol Cancer Ther. 2007 Feb;6(2):692-702. doi: 10.1158/1535-7163.MCT-06-0562. Mol Cancer Ther. 2007. PMID: 17308065
-
XIAP expression correlates with monocytic differentiation in adult de novo AML: impact on prognosis.Hematol J. 2004;5(6):489-95. doi: 10.1038/sj.thj.6200549. Hematol J. 2004. PMID: 15570290
-
XIAP antisense therapy with AEG 35156 in acute myeloid leukemia.Expert Opin Investig Drugs. 2013 May;22(5):663-70. doi: 10.1517/13543784.2013.789498. Expert Opin Investig Drugs. 2013. PMID: 23586880 Review.
-
Targeting BMP signaling in the bone marrow microenvironment of myeloid leukemia.Biochem Soc Trans. 2020 Apr 29;48(2):411-418. doi: 10.1042/BST20190223. Biochem Soc Trans. 2020. PMID: 32167132 Review.
Cited by
-
Repositioning of bromocriptine for treatment of acute myeloid leukemia.J Transl Med. 2016 Sep 7;14(1):261. doi: 10.1186/s12967-016-1007-5. J Transl Med. 2016. PMID: 27604463 Free PMC article.
-
MicroRNA-509-3p inhibits cell proliferation and invasion via downregulation of X-linked inhibitor of apoptosis in glioma.Oncol Lett. 2018 Jan;15(1):1307-1312. doi: 10.3892/ol.2017.7390. Epub 2017 Nov 10. Oncol Lett. 2018. PMID: 29399183 Free PMC article.
-
Destined to Die: Apoptosis and Pediatric Cancers.Cancers (Basel). 2019 Oct 23;11(11):1623. doi: 10.3390/cancers11111623. Cancers (Basel). 2019. PMID: 31652776 Free PMC article. Review.
-
miR-431-5p regulates cell proliferation and apoptosis in fibroblast-like synoviocytes in rheumatoid arthritis by targeting XIAP.Arthritis Res Ther. 2020 Oct 6;22(1):231. doi: 10.1186/s13075-020-02328-3. Arthritis Res Ther. 2020. PMID: 33023644 Free PMC article.
-
Emerging Therapies for Acute Myelogenus Leukemia Patients Targeting Apoptosis and Mitochondrial Metabolism.Cancers (Basel). 2019 Feb 22;11(2):260. doi: 10.3390/cancers11020260. Cancers (Basel). 2019. PMID: 30813354 Free PMC article. Review.
References
-
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellstrom-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. - PubMed
-
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. - PubMed
-
- Sachlos E, Risueno RM, Laronde S, Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn A, Graham M, Levadoux-Martin M, Lee JB, Giacomelli AO, Hassell JA, Fischer-Russell D, Trus MR, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149(6):1284–1297. - PubMed
-
- Zheng R, Friedman AD, Small D. Targeted inhibition of FLT3 overcomes the block to myeloid differentiation in 32Dcl3 cells caused by expression of FLT3/ITD mutations. Blood. 2002;100(12):4154–4161. - PubMed
-
- Petrie K, Zelent A, Waxman S. Differentiation therapy of acute myeloid leukemia: past, present and future. Curr Opin Hematol. 2009;16(2):84–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

