ADAM10 mediates trastuzumab resistance and is correlated with survival in HER2 positive breast cancer
- PMID: 24952873
- PMCID: PMC4196152
- DOI: 10.18632/oncotarget.1955
ADAM10 mediates trastuzumab resistance and is correlated with survival in HER2 positive breast cancer
Abstract
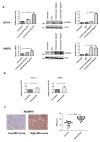
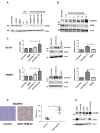
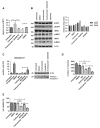
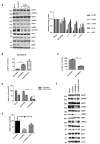
Trastuzumab prolongs survival in HER2 positive breast cancer patients. However, resistance remains a challenge. We have previously shown that ADAM17 plays a key role in maintaining HER2 phosphorylation during trastuzumab treatment. Beside ADAM17, ADAM10 is the other well characterized ADAM protease responsible for HER ligand shedding. Therefore, we studied the role of ADAM10 in relation to trastuzumab treatment and resistance in HER2 positive breast cancer. ADAM10 expression was assessed in HER2 positive breast cancer cell lines and xenograft mice treated with trastuzumab. Trastuzumab treatment increased ADAM10 levels in HER2 positive breast cancer cells (p ≤ 0.001 in BT474; p ≤ 0.01 in SKBR3) and in vivo (p ≤ 0.0001) compared to control, correlating with a decrease in PKB phosphorylation. ADAM10 inhibition or knockdown enhanced trastuzumab response in naïve and trastuzumab resistant breast cancer cells. Trastuzumab monotherapy upregulated ADAM10 (p ≤ 0.05); and higher pre-treatment ADAM10 levels correlated with decreased clinical response (p ≤ 0.05) at day 21 in HER2 positive breast cancer patients undergoing a trastuzumab treatment window study. Higher ADAM10 levels correlated with poorer relapse-free survival (p ≤ 0.01) in a cohort of HER2 positive breast cancer patients. Our studies implicate a role of ADAM10 in acquired resistance to trastuzumab and establish ADAM10 as a therapeutic target and a potential biomarker for HER2 positive breast cancer patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
ADAM10 and ADAM17: New players in trastuzumab tesistance.Oncotarget. 2014 Nov 30;5(22):10963-4. doi: 10.18632/oncotarget.2794. Oncotarget. 2014. PMID: 25460503 Free PMC article. No abstract available.
References
-
- Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–56. - PubMed
-
- Li SQ, Schmitz KR, Jeffrey PD, Wiltzius JJW, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–11. - PubMed
-
- Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–49. - PubMed
-
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. - PubMed
-
- Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

