FHL1C induces apoptosis in Notch1-dependent T-ALL cells through an interaction with RBP-J
- PMID: 24952875
- PMCID: PMC4077834
- DOI: 10.1186/1471-2407-14-463
FHL1C induces apoptosis in Notch1-dependent T-ALL cells through an interaction with RBP-J
Abstract
Background: Aberrantly activated Notch signaling has been found in more than 50% of patients with T-cell acute lymphoblastic leukemia (T-ALL). Current strategies that employ γ-secretase inhibitors (GSIs) to target Notch activation have not been successful. Many limitations, such as non-Notch specificity, dose-limiting gastrointestinal toxicity and GSI resistance, have prompted an urgent need for more effective Notch signaling inhibitors for T-ALL treatment. Human four-and-a-half LIM domain protein 1C (FHL1C) (KyoT2 in mice) has been demonstrated to suppress Notch activation in vitro, suggesting that FHL1C may be new candidate target in T-ALL therapy. However, the role of FHL1C in T-ALL cells remained unclear.
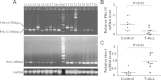
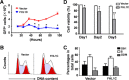
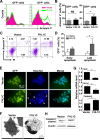
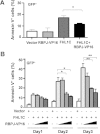
Methods: Using RT-PCR, we amplified full-length human FHL1C, and constructed full-length and various truncated forms of FHL1C. Using cell transfection, flow cytometry, transmission electron microscope, real-time RT-PCR, and Western blotting, we found that overexpression of FHL1C induced apoptosis of Jurkat cells. By using a reporter assay and Annexin-V staining, the minimal functional sequence of FHL1C inhibiting RBP-J-mediated Notch transactivation and inducing cell apoptosis was identified. Using real-time PCR and Western blotting, we explored the possible molecular mechanism of FHL1C-induced apoptosis. All data were statistically analyzed with the SPSS version 12.0 software.
Results: In Jurkat cells derived from a Notch1-associated T-ALL cell line insensitive to GSI treatment, we observed that overexpression of FHL1C, which is down-regulated in T-ALL patients, strongly induced apoptosis. Furthermore, we verified that FHL1C-induced apoptosis depended on the RBP-J-binding motif at the C-terminus of FHL1C. Using various truncated forms of FHL1C, we found that the RBP-J-binding motif of FHL1C had almost the same effect as full-length FHL1C on the induction of apoptosis, suggesting that the minimal functional sequence in the RBP-J-binding motif of FHL1C might be a new drug candidate for T-ALL treatment. We also explored the molecular mechanism of FHL1C overexpression-induced apoptosis, which suppressed downstream target genes such as Hes1 and c-Myc and key signaling pathways such as PI3K/AKT and NF-κB of Notch signaling involved in T-ALL progression.
Conclusions: Our study has revealed that FHL1C overexpression induces Jurkat cell apoptosis. This finding may provide new insights in designing new Notch inhibitors based on FHL1C to treat T-ALL.
Figures

References
-
- Wang L, Cheng T, Zheng G. The impact of tumor microenvironments on stem cells. Translational cancer research. 2013;2(5):422–428.
-
- Gridley T. Kick it up a notch: NOTCH1 activation in T-ALL. Cancer Cell. 2004;6(5):431–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

