Annotation of loci from genome-wide association studies using tissue-specific quantitative interaction proteomics
- PMID: 24952909
- PMCID: PMC4117722
- DOI: 10.1038/nmeth.2997
Annotation of loci from genome-wide association studies using tissue-specific quantitative interaction proteomics
Abstract
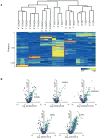
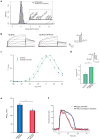
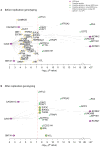
Genome-wide association studies (GWAS) have identified thousands of loci associated with complex traits, but it is challenging to pinpoint causal genes in these loci and to exploit subtle association signals. We used tissue-specific quantitative interaction proteomics to map a network of five genes involved in the Mendelian disorder long QT syndrome (LQTS). We integrated the LQTS network with GWAS loci from the corresponding common complex trait, QT-interval variation, to identify candidate genes that were subsequently confirmed in Xenopus laevis oocytes and zebrafish. We used the LQTS protein network to filter weak GWAS signals by identifying single-nucleotide polymorphisms (SNPs) in proximity to genes in the network supported by strong proteomic evidence. Three SNPs passing this filter reached genome-wide significance after replication genotyping. Overall, we present a general strategy to propose candidates in GWAS loci for functional studies and to systematically filter subtle association signals using tissue-specific quantitative interaction proteomics.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

