Enzyme replacement therapy in newborn mucopolysaccharidosis IVA mice: early treatment rescues bone lesions?
- PMID: 24953405
- PMCID: PMC4256128
- DOI: 10.1016/j.ymgme.2014.05.013
Enzyme replacement therapy in newborn mucopolysaccharidosis IVA mice: early treatment rescues bone lesions?
Abstract
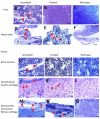
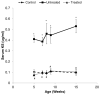
We treated mucopolysaccharidosis IVA (MPS IVA) mice to assess the effects of long-term enzyme replacement therapy (ERT) initiated at birth, since adult mice treated by ERT showed little improvement in bone pathology [1]. To conduct ERT in newborn mice, we used recombinant human N-acetylgalactosamine-6-sulfate sulfatase (GALNS) produced in a CHO cell line. First, to observe the tissue distribution pattern, a dose of 250units/g body weight was administered intravenously in MPS IVA mice at day 2 or 3. The infused enzyme was primarily recovered in the liver and spleen, with detectable activity in the bone and brain. Second, newborn ERT was conducted after a tissue distribution study. The first injection of newborn ERT was performed intravenously, the second to fourth weekly injections were intraperitoneal, and the remaining injections from 5th to 14th weeks were intravenous into the tail vein. MPS IVA mice treated with GALNS showed clearance of lysosomal storage in the liver and spleen, and sinus lining cells in bone marrow. The column structure of the growth plate was organized better than that in adult mice treated with ERT; however, hyaline and fibrous cartilage cells in the femur, spine, ligaments, discs, synovium, and periosteum still had storage materials to some extent. Heart valves were refractory to the treatment. Levels of serum keratan sulfate were kept normal in newborn ERT mice. In conclusion, the enzyme, which enters the cartilage before the cartilage cell layer becomes mature, prevents disorganization of column structure. Early treatment from birth leads to partial remission of bone pathology in MPS IVA mice.
Keywords: Enzyme replacement therapy; Fibrous cartilage; Hyaline cartilage; Mucopolysaccharidosis IVA; N-acetylgalactosamine-6-sulfate sulfatase.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Enzyme replacement in a human model of mucopolysaccharidosis IVA in vitro and its biodistribution in the cartilage of wild type mice.PLoS One. 2010 Aug 16;5(8):e12194. doi: 10.1371/journal.pone.0012194. PLoS One. 2010. PMID: 20808938 Free PMC article.
-
Enzyme replacement therapy in a murine model of Morquio A syndrome.Hum Mol Genet. 2008 Mar 15;17(6):815-24. doi: 10.1093/hmg/ddm353. Epub 2007 Dec 3. Hum Mol Genet. 2008. PMID: 18056156
-
Characterization and pharmacokinetic study of recombinant human N-acetylgalactosamine-6-sulfate sulfatase.Mol Genet Metab. 2007 May;91(1):69-78. doi: 10.1016/j.ymgme.2007.01.004. Epub 2007 Mar 2. Mol Genet Metab. 2007. PMID: 17336563
-
Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome.Drug Des Devel Ther. 2015 Apr 1;9:1937-53. doi: 10.2147/DDDT.S68562. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25897204 Free PMC article. Review.
-
Molecular genetics and metabolism, special edition: Diagnosis, diagnosis and prognosis of Mucopolysaccharidosis IVA.Mol Genet Metab. 2018 Sep;125(1-2):18-37. doi: 10.1016/j.ymgme.2018.05.004. Epub 2018 May 15. Mol Genet Metab. 2018. PMID: 29779902 Free PMC article. Review.
Cited by
-
Enzyme replacement therapy for treating mucopolysaccharidosis type IVA (Morquio A syndrome): effect and limitations.Expert Opin Orphan Drugs. 2015 Nov 1;3(11):1279-1290. doi: 10.1517/21678707.2015.1086640. Epub 2015 Oct 29. Expert Opin Orphan Drugs. 2015. PMID: 26973801 Free PMC article.
-
Adeno-Associated Virus Gene Transfer Ameliorates Progression of Skeletal Lesions in Mucopolysaccharidosis IVA Mice.Hum Gene Ther. 2024 Dec;35(23-24):955-968. doi: 10.1089/hum.2024.096. Epub 2024 Oct 25. Hum Gene Ther. 2024. PMID: 39450470
-
Sex Difference Leads to Differential Gene Expression Patterns and Therapeutic Efficacy in Mucopolysaccharidosis IVA Murine Model Receiving AAV8 Gene Therapy.Int J Mol Sci. 2022 Oct 21;23(20):12693. doi: 10.3390/ijms232012693. Int J Mol Sci. 2022. PMID: 36293546 Free PMC article.
-
Potential Targeting Mechanisms for Bone-Directed Therapies.Int J Mol Sci. 2024 Jul 30;25(15):8339. doi: 10.3390/ijms25158339. Int J Mol Sci. 2024. PMID: 39125906 Free PMC article. Review.
-
Biochemical evaluation of intracerebroventricular rhNAGLU-IGF2 enzyme replacement therapy in neonatal mice with Sanfilippo B syndrome.Mol Genet Metab. 2021 Jun;133(2):185-192. doi: 10.1016/j.ymgme.2021.03.013. Epub 2021 Mar 31. Mol Genet Metab. 2021. PMID: 33839004 Free PMC article.
References
-
- Tomatsu S, Montaño AM, Ohashi A, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–824. - PubMed
-
- Lowry RB, Applegarth DA, Toone JR, MacDonald E, Thunem NY. An update on the frequency of mucopolysaccharide syndromes in British Columbia. Hum. Genet. 1990;85:389–390. - PubMed
-
- Applegarth DA, Toone JR, Lowry RB. Incidence of inborn errors of metabolism in British Columbia. 1969–1996. Pediatrics. 2000;105:e10. - PubMed
-
- Nelson J. Incidence of the mucopolysaccharidoses in Northern Ireland. Hum Genet. 1997;101:355–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

