Green strategy from waste to value-added-chemical production: efficient biosynthesis of 6-hydroxy-3-succinoyl-pyridine by an engineered biocatalyst
- PMID: 24953905
- PMCID: PMC4066252
- DOI: 10.1038/srep05397
Green strategy from waste to value-added-chemical production: efficient biosynthesis of 6-hydroxy-3-succinoyl-pyridine by an engineered biocatalyst
Abstract
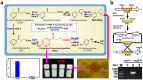
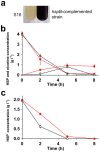
Value-added intermediates produced by microorganisms during the catabolism of N-heterocycles are potential building blocks for agrochemical synthesis and pharmaceutical production. 6-Hydroxy-3-succinoyl-pyridine (HSP), an intermediate in nicotine degradation, is an important precursor for the synthesis of drugs and compounds with biological activities. In the present study, we show that an engineered biocatalyst, Pseudomonas putida P-HSP, efficiently produced HSP from the renewable raw material of tobacco-waste that contains a high concentration of nicotine. The genetically constructed strain P-HSP realized a high accumulation of HSP, and HSP production was 3.7-fold higher than the non-engineered strain S16. Under optimal conditions, HSP was produced at high concentrations of 6.8 g l(-1) and 16.3 g l(-1) from tobacco-waste and nicotine, respectively. This work demonstrates a green strategy to block the catabolic pathway of N-heterocycles, which is a promising approach for the mutasynthesis of valuable compounds.
Figures

 ; nicotine,
; nicotine,  .
.

 ; nicotine,
; nicotine,  .
.
 ; nicotine,
; nicotine,  .
.Similar articles
-
Sustainable production of valuable compound 3-succinoyl-pyridine by genetically engineering Pseudomonas putida using the tobacco waste.Sci Rep. 2015 Nov 17;5:16411. doi: 10.1038/srep16411. Sci Rep. 2015. PMID: 26574178 Free PMC article.
-
"Green" route to 6-hydroxy-3-succinoyl-pyridine from (S)-nicotine of tobacco waste by whole cells of a Pseudomonas sp.Environ Sci Technol. 2005 Sep 1;39(17):6877-80. doi: 10.1021/es0500759. Environ Sci Technol. 2005. PMID: 16190252
-
Additional Role of Nicotinic Acid Hydroxylase for the Transformation of 3-Succinoyl-Pyridine by Pseudomonas sp. Strain JY-Q.Appl Environ Microbiol. 2021 Feb 26;87(6):e02740-20. doi: 10.1128/AEM.02740-20. Print 2021 Feb 26. Appl Environ Microbiol. 2021. PMID: 33397698 Free PMC article.
-
Nicotine-degrading microorganisms and their potential applications.Appl Microbiol Biotechnol. 2015 May;99(9):3775-85. doi: 10.1007/s00253-015-6525-1. Epub 2015 Mar 25. Appl Microbiol Biotechnol. 2015. PMID: 25805341 Review.
-
CO2 recycling: a key strategy to introduce green energy in the chemical production chain.ChemSusChem. 2014 May;7(5):1274-82. doi: 10.1002/cssc.201300926. Epub 2014 Mar 5. ChemSusChem. 2014. PMID: 24599714 Review.
Cited by
-
Microbial Degradation of Nicotinamide by a Strain Alcaligenes sp. P156.Sci Rep. 2019 Mar 6;9(1):3647. doi: 10.1038/s41598-019-40199-0. Sci Rep. 2019. PMID: 30842479 Free PMC article.
-
An enzymatic approach reverses nicotine dependence, decreases compulsive-like intake, and prevents relapse.Sci Adv. 2018 Oct 17;4(10):eaat4751. doi: 10.1126/sciadv.aat4751. eCollection 2018 Oct. Sci Adv. 2018. PMID: 30345354 Free PMC article.
-
Molecular mechanism of nicotine degradation by a newly isolated strain, Ochrobactrum sp. strain SJY1.Appl Environ Microbiol. 2015 Jan;81(1):272-81. doi: 10.1128/AEM.02265-14. Epub 2014 Oct 24. Appl Environ Microbiol. 2015. PMID: 25344232 Free PMC article.
-
A New Strategy for Smoking Cessation: Characterization of a Bacterial Enzyme for the Degradation of Nicotine.J Am Chem Soc. 2015 Aug 19;137(32):10136-9. doi: 10.1021/jacs.5b06605. Epub 2015 Aug 6. J Am Chem Soc. 2015. PMID: 26237398 Free PMC article.
-
Regulatory Mechanism of Nicotine Degradation in Pseudomonas putida.mBio. 2019 Jun 4;10(3):e00602-19. doi: 10.1128/mBio.00602-19. mBio. 2019. PMID: 31164460 Free PMC article.
References
-
- Schmid A. et al. Industrial biocatalysis today and tomorrow. Nature 409, 258–268 (2001). - PubMed
-
- Rathbone D. A. & Bruce N. C. Microbial transformation of alkaloids. Curr. Opin. Microbiol. 5, 274–281 (2002). - PubMed
-
- Forster-Carneiro T., Berni M., Dorileo I. & Rostagno M. Biorefinery study of availability of agriculture residues and wastes for integrated biorefineries in Brazil. Resour. Conserv. Recy. 77, 78–88 (2013).
-
- Wang J. H. et al. Bioaugmentation of activated sludge with Acinetobacter sp. TW enhances nicotine degradation in a synthetic tobacco wastewater treatment system. Bioresour. Technol. 142, 445–453 (2013). - PubMed
-
- Nanohar B. & Sridharan S. Extraction of nicotine from tobacco waste. Chem. Eng. World 22, 89–91 (1987).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

