Prenatal alcohol exposure alters expression of neurogenesis-related genes in an ex vivo cell culture model
- PMID: 24954023
- PMCID: PMC4096774
- DOI: 10.1016/j.alcohol.2014.06.001
Prenatal alcohol exposure alters expression of neurogenesis-related genes in an ex vivo cell culture model
Abstract
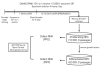
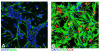
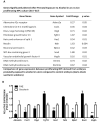
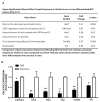
Prenatal alcohol exposure can lead to long-lasting changes in functional and genetic programs of the brain, which may underlie behavioral alterations seen in Fetal Alcohol Spectrum Disorder (FASD). Aberrant fetal programming during gestational alcohol exposure is a possible mechanism by which alcohol imparts teratogenic effects on the brain; however, current methods used to investigate the effects of alcohol on development often rely on either direct application of alcohol in vitro or acute high doses in vivo. In this study, we used our established moderate prenatal alcohol exposure (PAE) model, resulting in maternal blood alcohol content of approximately 20 mM, and subsequent ex vivo cell culture to assess expression of genes related to neurogenesis. Proliferating and differentiating neural progenitor cell culture conditions were established from telencephalic tissue derived from embryonic day (E) 15-17 tissue exposed to alcohol via maternal drinking throughout pregnancy. Gene expression analysis on mRNA derived in vitro was performed using a microarray, and quantitative PCR was conducted for genes to validate the microarray. Student's t tests were performed for statistical comparison of each exposure under each culture condition using a 95% confidence interval. Eleven percent of genes on the array had significantly altered mRNA expression in the prenatal alcohol-exposed neural progenitor culture under proliferating conditions. These include reduced expression of Adora2a, Cxcl1, Dlg4, Hes1, Nptx1, and Vegfa and increased expression of Fgf13, Ndn, and Sox3; bioinformatics analysis indicated that these genes are involved in cell growth and proliferation. Decreased levels of Dnmt1 and Dnmt3a were also found under proliferating conditions. Under differentiating conditions, 7.3% of genes had decreased mRNA expression; these include Cdk5rap3, Gdnf, Hey2, Heyl, Pard6b, and Ptn, which are associated with survival and differentiation as indicated by bioinformatics analysis. This study is the first to use chronic low to moderate PAE, to more accurately reflect maternal alcohol consumption, and subsequent neural progenitor cell culture to demonstrate that PAE throughout gestation alters expression of genes involved in neural development and embryonic neurogenesis.
Keywords: Alcohol; Cell culture; Chronic; DNA methylation; Development; Epigenetic; Gene expression; Neurogenesis; Prenatal.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Chronic Ethanol Exposure Alters DNA Methylation in Neural Stem Cells: Role of Mouse Strain and Sex.Mol Neurobiol. 2020 Feb;57(2):650-667. doi: 10.1007/s12035-019-01728-0. Epub 2019 Aug 14. Mol Neurobiol. 2020. PMID: 31414368
-
Prenatal exposure of ethanol induces increased glutamatergic neuronal differentiation of neural progenitor cells.J Biomed Sci. 2010 Nov 12;17(1):85. doi: 10.1186/1423-0127-17-85. J Biomed Sci. 2010. PMID: 21073715 Free PMC article.
-
A novel Oct4/Pou5f1-like non-coding RNA controls neural maturation and mediates developmental effects of ethanol.Neurotoxicol Teratol. 2021 Jan-Feb;83:106943. doi: 10.1016/j.ntt.2020.106943. Epub 2020 Nov 20. Neurotoxicol Teratol. 2021. PMID: 33221301 Free PMC article.
-
Long-term alterations to DNA methylation as a biomarker of prenatal alcohol exposure: From mouse models to human children with fetal alcohol spectrum disorders.Alcohol. 2017 May;60:67-75. doi: 10.1016/j.alcohol.2016.11.009. Epub 2016 Nov 22. Alcohol. 2017. PMID: 28187949 Review.
-
Neural crest development in fetal alcohol syndrome.Birth Defects Res C Embryo Today. 2014 Sep;102(3):210-20. doi: 10.1002/bdrc.21078. Epub 2014 Sep 15. Birth Defects Res C Embryo Today. 2014. PMID: 25219761 Free PMC article. Review.
Cited by
-
Effects of Genetics and Sex on Acute Gene Expression Changes in the Hippocampus Following Neonatal Ethanol Exposure in BXD Recombinant Inbred Mouse Strains.Brain Sci. 2022 Nov 29;12(12):1634. doi: 10.3390/brainsci12121634. Brain Sci. 2022. PMID: 36552094 Free PMC article.
-
Toxic and Teratogenic Effects of Prenatal Alcohol Exposure on Fetal Development, Adolescence, and Adulthood.Int J Mol Sci. 2021 Aug 16;22(16):8785. doi: 10.3390/ijms22168785. Int J Mol Sci. 2021. PMID: 34445488 Free PMC article. Review.
-
Chromatin Switches during Neural Cell Differentiation and Their Dysregulation by Prenatal Alcohol Exposure.Genes (Basel). 2017 May 11;8(5):137. doi: 10.3390/genes8050137. Genes (Basel). 2017. PMID: 28492482 Free PMC article. Review.
-
Prenatal arsenic exposure alters REST/NRSF and microRNA regulators of embryonic neural stem cell fate in a sex-dependent manner.Neurotoxicol Teratol. 2017 Jan-Feb;59:1-15. doi: 10.1016/j.ntt.2016.10.004. Epub 2016 Oct 14. Neurotoxicol Teratol. 2017. PMID: 27751817 Free PMC article.
-
Environmental Influences on Genomic Imprinting.Curr Environ Health Rep. 2015 Jun;2(2):155-62. doi: 10.1007/s40572-015-0046-z. Curr Environ Health Rep. 2015. PMID: 26029493 Free PMC article. Review.
References
-
- Aoto K, Shikata Y, Higashiyama D, Shiota K, Motoyama J. Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Research. Part A, Clinical and Molecular Teratology. 2008;82:224–231. - PubMed
-
- Barton A, Fendrik AJ. Sustained vs. oscillating expressions of Ngn2, Dll1 and Hes1: a model of neural differentiation of embryonic telencephalon. Journal of Theoretical Biology. 2013;328:1–8. - PubMed
-
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nature Neuroscience. 2003;6:1162–1168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

