Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma
- PMID: 24954133
- PMCID: PMC4441014
- DOI: 10.1016/j.ccr.2014.05.005
Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma
Abstract
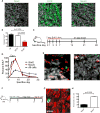
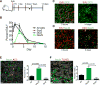
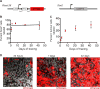
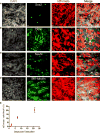
Functional heterogeneity within tumors presents a significant therapeutic challenge. Here we show that quiescent, therapy-resistant Sox2(+) cells propagate sonic hedgehog subgroup medulloblastoma by a mechanism that mirrors a neurogenic program. Rare Sox2(+) cells produce rapidly cycling doublecortin(+) progenitors that, together with their postmitotic progeny expressing NeuN, comprise tumor bulk. Sox2(+) cells are enriched following anti-mitotic chemotherapy and Smoothened inhibition, creating a reservoir for tumor regrowth. Lineage traces from Sox2(+) cells increase following treatment, suggesting that this population is responsible for relapse. Targeting Sox2(+) cells with the antineoplastic mithramycin abrogated tumor growth. Addressing functional heterogeneity and eliminating Sox2(+) cells presents a promising therapeutic paradigm for treatment of sonic hedgehog subgroup medulloblastoma.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Sox2: masterminding the root of cancer.Cancer Cell. 2014 Jul 14;26(1):3-5. doi: 10.1016/j.ccr.2014.06.024. Cancer Cell. 2014. PMID: 25026204
-
Sox2, a marker for stem-like tumor cells in skin squamous cell carcinoma and hedgehog subgroup medulloblastoma.EMBO J. 2014 Sep 17;33(18):1984-6. doi: 10.15252/embj.201489479. Epub 2014 Jul 24. EMBO J. 2014. PMID: 25061226 Free PMC article.
References
-
- Ahlfeld J, Favaro R, Pagella P, Kretzschmar HA, Nicolis S, Schüller U. Sox2 requirement in sonic hedgehog-associated medulloblastoma. Cancer Res. 2013;73:3796–3807. - PubMed
-
- Bailey P, Cushing H. Medulloblastoma cerebelli: A common type of midcerebellar glioma of childhood. Arch Neurol Psychiatry. 1925;14:192–224.
-
- Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

