Treatment of chronically FIV-infected cats with suberoylanilide hydroxamic acid
- PMID: 24954265
- PMCID: PMC4119798
- DOI: 10.1016/j.antiviral.2014.05.014
Treatment of chronically FIV-infected cats with suberoylanilide hydroxamic acid
Abstract
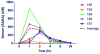
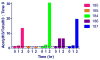
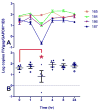
Feline immunodeficiency virus (FIV) is a naturally-occurring, large animal model of lentiviral-induced immunodeficiency syndrome, and has been used as a model of HIV pathogenesis and therapeutic interventions. HIV reservoirs in the form of latent virus remain the primary roadblock to viral eradication and cure, and FIV has been previously established an animal model of lentiviral latency. The goal of this study was to determine whether administration of the histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA) to aviremic, chronically FIV-infected cats would induce latent viral reactivation in vivo. A proof-of-concept experiment in a Transwell co-culture system demonstrated the ability of SAHA to reactivate latent virus which was replication competent and able to infect naïve cells. Oral SAHA (250mg/m(2)) was administered with food to four asymptomatic, experimentally FIV-infected cats and one uninfected control cat, and a limited pharmacokinetic and pharmacodynamic analysis was performed. A statistically significant increase in cell-associated FIV RNA was detected in the cat with the greatest serum SAHA exposure, and cell-free viral RNA was detected at one time point in the three cats that achieved the highest levels of SAHA in serum. Interestingly, there was a significant decrease in viral DNA burden at 2h post drug administration in the same three cats. Though the sample size is small and the drug response was modest, this study provides evidence that in vivo treatment of FIV-infected cats with the HDACi SAHA can induce viral transcriptional reactivation, which may be dependent upon the concentration of SAHA achieved in blood. Importantly, alternative putative antilatency therapy drugs, and multimodal drug combinations, could be studied in this in vivo system. The FIV/cat model provides a unique opportunity to test novel therapeutic interventions aimed at eradicating latent virus in vivo.
Keywords: Animal model; Antilatency therapy; FIV; HDAC inhibitor; HIV; SAHA.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures
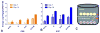
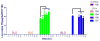
Similar articles
-
Viral Reservoirs in Lymph Nodes of FIV-Infected Progressor and Long-Term Non-Progressor Cats during the Asymptomatic Phase.PLoS One. 2016 Jan 7;11(1):e0146285. doi: 10.1371/journal.pone.0146285. eCollection 2016. PLoS One. 2016. PMID: 26741651 Free PMC article.
-
Pharmacologic reactivation of latent feline immunodeficiency virus ex vivo in peripheral CD4+ T-lymphocytes.Virus Res. 2012 Dec;170(1-2):174-9. doi: 10.1016/j.virusres.2012.10.009. Epub 2012 Oct 13. Virus Res. 2012. PMID: 23073179 Free PMC article.
-
Transcriptional regulation of latent feline immunodeficiency virus in peripheral CD4+ T-lymphocytes.Viruses. 2012 May;4(5):878-88. doi: 10.3390/v4050878. Epub 2012 May 23. Viruses. 2012. PMID: 22754653 Free PMC article.
-
Feline immunodeficiency virus latency.Retrovirology. 2013 Jul 6;10:69. doi: 10.1186/1742-4690-10-69. Retrovirology. 2013. PMID: 23829177 Free PMC article. Review.
-
Neurologic disease in feline immunodeficiency virus infection: disease mechanisms and therapeutic interventions for NeuroAIDS.J Neurovirol. 2018 Apr;24(2):220-228. doi: 10.1007/s13365-017-0593-1. Epub 2017 Dec 15. J Neurovirol. 2018. PMID: 29247305 Review.
Cited by
-
Multiple, Independent T Cell Lymphomas Arising in an Experimentally FIV-Infected Cat during the Terminal Stage of Infection.Viruses. 2018 May 24;10(6):280. doi: 10.3390/v10060280. Viruses. 2018. PMID: 29794987 Free PMC article.
-
Histone Deacetylase Inhibitors and Microtubule Inhibitors Induce Apoptosis in Feline Luminal Mammary Carcinoma Cells.Animals (Basel). 2021 Feb 15;11(2):502. doi: 10.3390/ani11020502. Animals (Basel). 2021. PMID: 33671894 Free PMC article.
-
Animal Models for HIV Cure Research.Front Immunol. 2016 Jan 28;7:12. doi: 10.3389/fimmu.2016.00012. eCollection 2016. Front Immunol. 2016. PMID: 26858716 Free PMC article. Review.
-
Follow-Up of Viral Parameters in FeLV- or FIV-Naturally Infected Cats Treated Orally with Low Doses of Human Interferon Alpha.Viruses. 2019 Sep 11;11(9):845. doi: 10.3390/v11090845. Viruses. 2019. PMID: 31514435 Free PMC article.
-
Viral Reservoirs in Lymph Nodes of FIV-Infected Progressor and Long-Term Non-Progressor Cats during the Asymptomatic Phase.PLoS One. 2016 Jan 7;11(1):e0146285. doi: 10.1371/journal.pone.0146285. eCollection 2016. PLoS One. 2016. PMID: 26741651 Free PMC article.
References
-
- Archin NM, Bateson R, Tripathy M, Crooks AM, Yang KH, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, Richman DD, Robertson KR, Kashuba AD, Bosch RJ, Hazuda DJ, Kuruc JD, Eron JJ, Margolis DM. HIV-1 Expression within Resting CD4 T-Cells Following Multiple Doses of Vorinostat. J Infect Dis 2014 - PMC - PubMed
-
- Bisset LR, Lutz H, Boni J, Hofmann-Lehmann R, Luthy R, Schupbach J. Combined effect of zidovudine (ZDV), lamivudine (3TC) and abacavir (ABC) antiretroviral therapy in suppressing in vitro FIV replication. Antiviral Res. 2002;53:35–45. - PubMed
-
- Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. - PubMed
-
- Burkhard MJ, Dean GA. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr HIV Res. 2003;1:15–29. - PubMed
-
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

