The LYR factors SDHAF1 and SDHAF3 mediate maturation of the iron-sulfur subunit of succinate dehydrogenase
- PMID: 24954417
- PMCID: PMC4126850
- DOI: 10.1016/j.cmet.2014.05.014
The LYR factors SDHAF1 and SDHAF3 mediate maturation of the iron-sulfur subunit of succinate dehydrogenase
Abstract
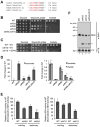
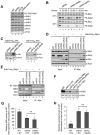
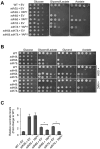
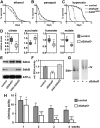
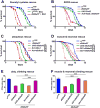
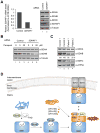
Disorders arising from impaired assembly of succinate dehydrogenase (SDH) result in a myriad of pathologies, consistent with its unique role in linking the citric acid cycle and electron transport chain. In spite of this critical function, however, only a few factors are known to be required for SDH assembly and function. We show here that two factors, Sdh6 (SDHAF1) and Sdh7 (SDHAF3), mediate maturation of the FeS cluster SDH subunit (Sdh2/SDHB). Yeast and Drosophila lacking SDHAF3 are impaired in SDH activity with reduced levels of Sdh2. Drosophila lacking the Sdh7 ortholog SDHAF3 are hypersensitive to oxidative stress and exhibit muscular and neuronal dysfunction. Yeast studies revealed that Sdh6 and Sdh7 act together to promote Sdh2 maturation by binding to a Sdh1/Sdh2 intermediate, protecting it from the deleterious effects of oxidants. These studies in yeast and Drosophila raise the possibility that SDHAF3 mutations may be associated with idiopathic SDH-associated diseases.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Analysis of SDHAF3 in familial and sporadic pheochromocytoma and paraganglioma.BMC Cancer. 2017 Jul 24;17(1):497. doi: 10.1186/s12885-017-3486-z. BMC Cancer. 2017. PMID: 28738844 Free PMC article.
-
SDHAF4 promotes mitochondrial succinate dehydrogenase activity and prevents neurodegeneration.Cell Metab. 2014 Aug 5;20(2):241-52. doi: 10.1016/j.cmet.2014.05.012. Epub 2014 Jun 19. Cell Metab. 2014. PMID: 24954416 Free PMC article.
-
The mitochondrial LYR protein SDHAF1 is required for succinate dehydrogenase activity in Arabidopsis.Plant J. 2022 Apr;110(2):499-512. doi: 10.1111/tpj.15684. Epub 2022 Feb 26. Plant J. 2022. PMID: 35080330 Free PMC article.
-
The assembly of succinate dehydrogenase: a key enzyme in bioenergetics.Cell Mol Life Sci. 2019 Oct;76(20):4023-4042. doi: 10.1007/s00018-019-03200-7. Epub 2019 Jun 24. Cell Mol Life Sci. 2019. PMID: 31236625 Free PMC article. Review.
-
Sequence diversity and conservation in factors influencing succinate dehydrogenase flavinylation.Plant Signal Behav. 2013 Feb;8(2):e22815. doi: 10.4161/psb.22815. Epub 2012 Nov 15. Plant Signal Behav. 2013. PMID: 23154507 Free PMC article. Review.
Cited by
-
Plasma extracellular vesicle long RNA profiling identifies a predictive signature for immunochemotherapy efficacy in lung squamous cell carcinoma.Front Immunol. 2024 Aug 5;15:1421604. doi: 10.3389/fimmu.2024.1421604. eCollection 2024. Front Immunol. 2024. PMID: 39161762 Free PMC article.
-
Maturation of the respiratory complex II flavoprotein.Curr Opin Struct Biol. 2019 Dec;59:38-46. doi: 10.1016/j.sbi.2019.01.027. Epub 2019 Mar 7. Curr Opin Struct Biol. 2019. PMID: 30851631 Free PMC article. Review.
-
Cancer mutational burden is shaped by G4 DNA, replication stress and mitochondrial dysfunction.Prog Biophys Mol Biol. 2019 Oct;147:47-61. doi: 10.1016/j.pbiomolbio.2019.03.004. Epub 2019 Mar 14. Prog Biophys Mol Biol. 2019. PMID: 30880007 Free PMC article.
-
Blackout in the powerhouse: clinical phenotypes associated with defects in the assembly of OXPHOS complexes and the mitoribosome.Biochem J. 2020 Nov 13;477(21):4085-4132. doi: 10.1042/BCJ20190767. Biochem J. 2020. PMID: 33151299 Free PMC article. Review.
-
Analysis of transcriptional profiles of Saccharomyces cerevisiae exposed to bisphenol A.Curr Genet. 2017 May;63(2):253-274. doi: 10.1007/s00294-016-0633-z. Epub 2016 Jul 26. Curr Genet. 2017. PMID: 27460658
References
-
- Bardella C, Pollard PJ, Tomlinson I. SDH mutations in cancer. Biochim Biophys Acta. 2011;1807:1432–1443. - PubMed
-
- Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers, et al. Mutations in SDHD, a mitochondrial complex II gene in hereditary paraganglioma. Science. 2000;287:848–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

