High density lipoprotein metabolism in low density lipoprotein receptor-deficient mice
- PMID: 24954421
- PMCID: PMC4617360
- DOI: 10.1194/jlr.M048819
High density lipoprotein metabolism in low density lipoprotein receptor-deficient mice
Abstract
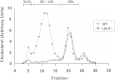
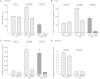
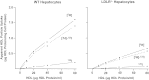
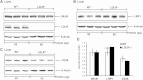
The LDL receptor (LDLR) and scavenger receptor class B type I (SR-BI) play physiological roles in LDL and HDL metabolism in vivo. In this study, we explored HDL metabolism in LDLR-deficient mice in comparison with WT littermates. Murine HDL was radiolabeled in the protein ((125)I) and in the cholesteryl ester (CE) moiety ([(3)H]). The metabolism of (125)I-/[(3)H]HDL was investigated in plasma and in tissues of mice and in murine hepatocytes. In WT mice, liver and adrenals selectively take up HDL-associated CE ([(3)H]). In contrast, in LDLR(-/-) mice, selective HDL CE uptake is significantly reduced in liver and adrenals. In hepatocytes isolated from LDLR(-/-) mice, selective HDL CE uptake is substantially diminished compared with WT liver cells. Hepatic and adrenal protein expression of lipoprotein receptors SR-BI, cluster of differentiation 36 (CD36), and LDL receptor-related protein 1 (LRP1) was analyzed by immunoblots. The respective protein levels were identical both in hepatic and adrenal membranes prepared from WT or from LDLR(-/-) mice. In summary, an LDLR deficiency substantially decreases selective HDL CE uptake by liver and adrenals. This decrease is independent from regulation of receptor proteins like SR-BI, CD36, and LRP1. Thus, LDLR expression has a substantial impact on both HDL and LDL metabolism in mice.
Keywords: cholesteryl ester; scavenger receptor class B type I; selective uptake.
Copyright © 2014 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
The role of human and mouse hepatic scavenger receptor class B type I (SR-BI) in the selective uptake of low-density lipoprotein-cholesteryl esters.Biochemistry. 2003 Jun 24;42(24):7527-38. doi: 10.1021/bi026949a. Biochemistry. 2003. PMID: 12809509
-
Scavenger receptor class B type I mediates the selective uptake of high-density lipoprotein-associated cholesteryl ester by the liver in mice.Arterioscler Thromb Vasc Biol. 2005 Jan;25(1):143-8. doi: 10.1161/01.ATV.0000149381.16166.c6. Epub 2004 Nov 4. Arterioscler Thromb Vasc Biol. 2005. PMID: 15528479
-
Upregulation of selective cholesteryl ester uptake pathway in mice with deletion of low-density lipoprotein receptor function.J Cell Physiol. 1999 Aug;180(2):190-202. doi: 10.1002/(SICI)1097-4652(199908)180:2<190::AID-JCP7>3.0.CO;2-Z. J Cell Physiol. 1999. PMID: 10395289
-
Roles of scavenger receptor BI and APO A-I in selective uptake of HDL cholesterol by adrenal cells.Endocr Res. 2000 Nov;26(4):639-51. doi: 10.3109/07435800009048584. Endocr Res. 2000. PMID: 11196441 Review.
-
Foam cells in atherosclerosis.Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review.
Cited by
-
NR4A1 Deletion in Marginal Zone B Cells Exacerbates Atherosclerosis in Mice-Brief Report.Arterioscler Thromb Vasc Biol. 2020 Nov;40(11):2598-2604. doi: 10.1161/ATVBAHA.120.314607. Epub 2020 Sep 10. Arterioscler Thromb Vasc Biol. 2020. PMID: 32907369 Free PMC article.
-
FoxO transcription factors are required for hepatic HDL cholesterol clearance.J Clin Invest. 2018 Apr 2;128(4):1615-1626. doi: 10.1172/JCI94230. Epub 2018 Mar 19. J Clin Invest. 2018. PMID: 29408809 Free PMC article.
-
Genetic alterations affecting cholesterol metabolism and human fertility.Biol Reprod. 2014 Nov;91(5):117. doi: 10.1095/biolreprod.114.119883. Epub 2014 Aug 13. Biol Reprod. 2014. PMID: 25122065 Free PMC article. Review.
-
Reverse Cholesterol Transport Dysfunction Is a Feature of Familial Hypercholesterolemia.Curr Atheroscler Rep. 2021 Apr 29;23(6):29. doi: 10.1007/s11883-021-00928-1. Curr Atheroscler Rep. 2021. PMID: 33914189 Review.
-
Targeted Deletion of Hepatocyte Abca1 Increases Plasma HDL (High-Density Lipoprotein) Reverse Cholesterol Transport via the LDL (Low-Density Lipoprotein) Receptor.Arterioscler Thromb Vasc Biol. 2019 Sep;39(9):1747-1761. doi: 10.1161/ATVBAHA.119.312382. Epub 2019 Jun 6. Arterioscler Thromb Vasc Biol. 2019. PMID: 31167565 Free PMC article.
References
-
- Eisenberg S. 1984. High density lipoprotein metabolism. J. Lipid Res. 25: 1017–1058. - PubMed
-
- Brown M. S., Goldstein J. L. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47. - PubMed
-
- Carew T. E., Pittman R. C., Steinberg D. 1982. Tissue sites of degradation of native and reductively methylated [14C]sucrose-labeled low density lipoprotein in rats. J. Biol. Chem. 257: 8001–8008. - PubMed
-
- Acton S., Rigotti A., Landschulz K. T., Xu S., Hobbs H. H., Krieger M. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 271: 518–520. - PubMed
-
- Glass C., Pittman R. C., Civen M., Steinberg D. 1985. Uptake of high-density lipoprotein-associated apoprotein A-I and cholesterol esters by 16 tissues of the rat in vivo and by adrenal cells and hepatocytes in vitro. J. Biol. Chem. 260: 744–750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

