Targeted inhibition of prostate cancer metastases with an RNA aptamer to prostate-specific membrane antigen
- PMID: 24954476
- PMCID: PMC4429728
- DOI: 10.1038/mt.2014.117
Targeted inhibition of prostate cancer metastases with an RNA aptamer to prostate-specific membrane antigen
Abstract
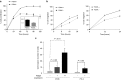
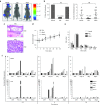
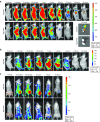
Cell-targeted therapies (smart drugs), which selectively control cancer cell progression with limited toxicity to normal cells, have been developed to effectively treat some cancers. However, many cancers such as metastatic prostate cancer (PC) have yet to be treated with current smart drug technology. Here, we describe the thorough preclinical characterization of an RNA aptamer (A9g) that functions as a smart drug for PC by inhibiting the enzymatic activity of prostate-specific membrane antigen (PSMA). Treatment of PC cells with A9g results in reduced cell migration/invasion in culture and metastatic disease in vivo. Importantly, A9g is safe in vivo and is not immunogenic in human cells. Pharmacokinetic and biodistribution studies in mice confirm target specificity and absence of non-specific on/off-target effects. In conclusion, these studies provide new and important insights into the role of PSMA in driving carcinogenesis and demonstrate critical endpoints for the translation of a novel RNA smart drug for advanced stage PC.
Figures


Comment in
-
A theranostic "SMART" aptamer for targeted therapy of prostate cancer.Mol Ther. 2014 Nov;22(11):1886-8. doi: 10.1038/mt.2014.190. Mol Ther. 2014. PMID: 25365986 Free PMC article. No abstract available.
References
-
- Cereda V, Formica V, Massimiani G, Tosetto L, Roselli M. Targeting metastatic castration-resistant prostate cancer: mechanisms of progression and novel early therapeutic approaches. Expert Opin Investig Drugs. 2014;23:469–487. - PubMed
-
- Shah N, Dizon DS. New-generation platinum agents for solid tumors. Future Oncol. 2009;5:33–42. - PubMed
-
- Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. - PubMed
-
- Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54:1807–1811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

