Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort
- PMID: 24954778
- PMCID: PMC4142216
- DOI: 10.1016/S1470-2045(14)70265-7
Risk of late effects of treatment in children newly diagnosed with standard-risk acute lymphoblastic leukaemia: a report from the Childhood Cancer Survivor Study cohort
Abstract
Background: Treatment of patients with paediatric acute lymphoblastic leukaemia has evolved such that the risk of late effects in survivors treated in accordance with contemporary protocols could be different from that noted in those treated decades ago. We aimed to estimate the risk of late effects in children with standard-risk acute lymphoblastic leukaemia treated with contemporary protocols.
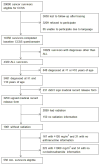
Methods: We used data from similarly treated members of the Childhood Cancer Survivor Study cohort. The Childhood Cancer Survivor Study is a multicentre, North American study of 5-year survivors of childhood cancer diagnosed between 1970 and 1986. We included cohort members if they were aged 1·0-9·9 years at the time of diagnosis of acute lymphoblastic leukaemia and had received treatment consistent with contemporary standard-risk protocols for acute lymphoblastic leukaemia. We calculated mortality rates and standardised mortality ratios, stratified by sex and survival time, after diagnosis of acute lymphoblastic leukaemia. We calculated standardised incidence ratios and absolute excess risk for subsequent neoplasms with age-specific, sex-specific, and calendar-year-specific rates from the Surveillance, Epidemiology and End Results Program. Outcomes were compared with a sibling cohort and the general US population.
Findings: We included 556 (13%) of 4329 cohort members treated for acute lymphoblastic leukaemia. Median follow-up of the survivors from 5 years after diagnosis was 18·4 years (range 0·0-33·0). 28 (5%) of 556 participants had died (standardised mortality ratio 3·5, 95% CI 2·3-5·0). 16 (57%) deaths were due to causes other than recurrence of acute lymphoblastic leukaemia. Six (1%) survivors developed a subsequent malignant neoplasm (standardised incidence ratio 2·6, 95% CI 1·0-5·7). 107 participants (95% CI 81-193) in each group would need to be followed-up for 1 year to observe one extra chronic health disorder in the survivor group compared with the sibling group. 415 participants (376-939) in each group would need to be followed-up for 1 year to observe one extra severe, life-threatening, or fatal disorder in the group of survivors. Survivors did not differ from siblings in their educational attainment, rate of marriage, or independent living.
Interpretation: The prevalence of adverse long-term outcomes in children treated for standard risk acute lymphoblastic leukaemia according to contemporary protocols is low, but regular care from a knowledgeable primary-care practitioner is warranted.
Funding: National Cancer Institute, American Lebanese-Syrian Associated Charities, Swiss Cancer Research.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures
Comment in
-
How safe is a standard-risk child with ALL?Lancet Oncol. 2014 Jul;15(8):782-3. doi: 10.1016/S1470-2045(14)70294-3. Epub 2014 Jun 19. Lancet Oncol. 2014. PMID: 24954780 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical