Dual-Emissive Difluoroboron Naphthyl-Phenyl β-Diketonate Polylactide Materials: Effects of Heavy Atom Placement and Polymer Molecular Weight
- PMID: 24954954
- PMCID: PMC4059218
- DOI: 10.1021/ma5006606
Dual-Emissive Difluoroboron Naphthyl-Phenyl β-Diketonate Polylactide Materials: Effects of Heavy Atom Placement and Polymer Molecular Weight
Abstract
Luminescent materials are important for imaging and sensing. Aromatic difluoroboron β-diketonate complexes (BF2bdks) are classic fluorescent molecules that have been explored as photochemical reagents, two-photon dyes, and oxygen sensors. A series of BF2bdks with naphthyl and phenyl groups was synthesized, and photophysical properties were investigated in both methylene chloride and poly(lactic acid) (PLA). Polymer molecular weight and dye attachment site along with bromide heavy atom placement were varied to tune optical properties of dye-PLA materials. Systems without heavy atoms have long phosphorescence lifetimes, which is useful for lifetime-based oxygen sensing. Bromine substitution on the naphthyl ring resulted in intense, clearly distinguishable fluorescence and phosphorescence peaks important for ratiometric oxygen sensing and imaging.
Figures





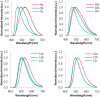

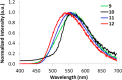
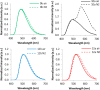
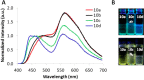
Similar articles
-
Oxygen Sensing Difluoroboron Dinaphthoylmethane Polylactide.Macromolecules. 2015 May 12;48(9):2967-2977. doi: 10.1021/acs.macromol.5b00394. Macromolecules. 2015. PMID: 26056421 Free PMC article.
-
Tailoring Oxygen Sensitivity with Halide Substitution in Difluoroboron Dibenzoylmethane Polylactide Materials.ACS Appl Mater Interfaces. 2015 Oct 28;7(42):23633-43. doi: 10.1021/acsami.5b07126. Epub 2015 Oct 19. ACS Appl Mater Interfaces. 2015. PMID: 26480236 Free PMC article.
-
Aromatic difluoroboron β-diketonate complexes: effects of π-conjugation and media on optical properties.Inorg Chem. 2013 Apr 1;52(7):3597-610. doi: 10.1021/ic300077g. Epub 2013 Mar 19. Inorg Chem. 2013. PMID: 23510181 Free PMC article.
-
Visible-light sensitized luminescent europium(III)-β-diketonate complexes: bioprobes for cellular imaging.Dalton Trans. 2013 Nov 21;42(43):15249-62. doi: 10.1039/c3dt52238e. Dalton Trans. 2013. PMID: 24076753 Review.
-
Ratiometric fluorescence, electrochemiluminescence, and photoelectrochemical chemo/biosensing based on semiconductor quantum dots.Nanoscale. 2016 Apr 28;8(16):8427-42. doi: 10.1039/c6nr01912a. Epub 2016 Apr 8. Nanoscale. 2016. PMID: 27056088 Review.
Cited by
-
Oxygen Sensing Difluoroboron Dinaphthoylmethane Polylactide.Macromolecules. 2015 May 12;48(9):2967-2977. doi: 10.1021/acs.macromol.5b00394. Macromolecules. 2015. PMID: 26056421 Free PMC article.
-
Oxygen Sensing Difluoroboron β-Diketonate Polylactide Materials with Tunable Dynamic Ranges for Wound Imaging.ACS Sens. 2016 Nov 23;1(11):1366-1373. doi: 10.1021/acssensors.6b00533. Epub 2016 Nov 14. ACS Sens. 2016. PMID: 28042606 Free PMC article.
-
Tailoring Oxygen Sensitivity with Halide Substitution in Difluoroboron Dibenzoylmethane Polylactide Materials.ACS Appl Mater Interfaces. 2015 Oct 28;7(42):23633-43. doi: 10.1021/acsami.5b07126. Epub 2015 Oct 19. ACS Appl Mater Interfaces. 2015. PMID: 26480236 Free PMC article.
-
Thienyl Difluoroboron β-Diketonates in Solution and Polylactide Media.Aust J Chem. 2016;69(5):537-545. doi: 10.1071/CH15750. Epub 2016 Mar 7. Aust J Chem. 2016. PMID: 27833174 Free PMC article.
-
Halide-containing organic persistent luminescent materials for environmental sensing applications.Chem Sci. 2021 Dec 20;13(8):2184-2201. doi: 10.1039/d1sc06586f. eCollection 2022 Feb 23. Chem Sci. 2021. PMID: 35310490 Free PMC article. Review.
References
-
- Wang X. D.; Wolfbeis O. S.. Chem. Soc. Rev. 2014, ASAP
-
- Meier R. J.; Schreml S.; Wang X. D.; Landthaler M.; Babilas P.; Wolfbeis O. S. Angew. Chem., Int. Ed. 2011, 50, 10893–10896. - PubMed
-
- Wang X. D.; Gorris H. H.; Stolwijk J. A.; Meier R. J.; Groegel D. B. M.; Wegener J.; Wolfbeis O. S. Chem. Sci. 2011, 2, 901–906.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
