Polymorphic Protein Crystal Growth: Influence of Hydration and Ions in Glucose Isomerase
- PMID: 24955067
- PMCID: PMC4061714
- DOI: 10.1021/cg401063b
Polymorphic Protein Crystal Growth: Influence of Hydration and Ions in Glucose Isomerase
Abstract
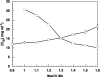
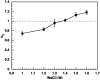
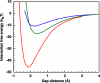
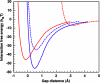
Crystal polymorphs of glucose isomerase were examined to characterize the properties and to quantify the energetics of protein crystal growth. Transitions of polymorph stability were measured in poly(ethylene glycol)/NaCl solutions, and one transition point was singled out for more detailed quantitative analysis. Single crystal x-ray diffraction was used to confirm space groups and identify complementary crystal structures. Crystal polymorph stability was found to depend on the NaCl concentration, with stability transitions requiring > 1 M NaCl combined with a low concentration of PEG. Both salting-in and salting-out behavior was observed and was found to differ for the two polymorphs. For NaCl concentrations above the observed polymorph transition, the increase in solubility of the less stable polymorph together with an increase in the osmotic second virial coefficient suggests that changes in protein hydration upon addition of salt may explain the experimental trends. A combination of atomistic and continuum models was employed to dissect this behavior. Molecular dynamics simulations of the solvent environment were interpreted using quasi-chemical theory to understand changes in protein hydration as a function of NaCl concentration. The results suggest that protein surface hydration and Na+ binding may introduce steric barriers to contact formation, resulting in polymorph selection.
Figures

Similar articles
-
Polymorphism in Spin Transition Systems. Crystal Structure, Magnetic Properties, and Mössbauer Spectroscopy of Three Polymorphic Modifications of [Fe(DPPA)(NCS)(2)] [DPPA = (3-Aminopropyl)bis(2-pyridylmethyl)amine].Inorg Chem. 1997 Dec 3;36(25):5869-5879. doi: 10.1021/ic971174t. Inorg Chem. 1997. PMID: 11670210
-
Understanding the Influence of Surface Solvation and Structure on Polymorph Stability: A Combined Mechanochemical and Theoretical Approach.J Am Chem Soc. 2018 Dec 12;140(49):17051-17059. doi: 10.1021/jacs.8b08549. Epub 2018 Oct 29. J Am Chem Soc. 2018. PMID: 30371073
-
Stabilization of halophilic malate dehydrogenase.J Mol Biol. 1989 Aug 5;208(3):491-500. doi: 10.1016/0022-2836(89)90512-3. J Mol Biol. 1989. PMID: 2795658
-
Polymorphic phase transitions: Macroscopic theory and molecular simulation.Adv Drug Deliv Rev. 2017 Aug 1;117:47-70. doi: 10.1016/j.addr.2017.09.017. Epub 2017 Sep 20. Adv Drug Deliv Rev. 2017. PMID: 28939378 Review.
-
Polymorphic Behavior and Phase Transition of Poly(1-Butene) and Its Copolymers.Polymers (Basel). 2018 May 21;10(5):556. doi: 10.3390/polym10050556. Polymers (Basel). 2018. PMID: 30966590 Free PMC article. Review.
Cited by
-
Observing classical nucleation theory at work by monitoring phase transitions with molecular precision.Nat Commun. 2014 Dec 3;5:5598. doi: 10.1038/ncomms6598. Nat Commun. 2014. PMID: 25465441 Free PMC article.
-
Diverse crystalline protein scaffolds through metal-dependent polymorphism.Protein Sci. 2024 May;33(5):e4971. doi: 10.1002/pro.4971. Protein Sci. 2024. PMID: 38591647 Free PMC article.
-
Nucleation of glucose isomerase protein crystals in a nonclassical disguise: The role of crystalline precursors.Proc Natl Acad Sci U S A. 2022 Feb 15;119(7):e2108674119. doi: 10.1073/pnas.2108674119. Proc Natl Acad Sci U S A. 2022. PMID: 35101915 Free PMC article.
-
Variability in the Spatial Structure of the Central Loop in Cobra Cytotoxins Revealed by X-ray Analysis and Molecular Modeling.Toxins (Basel). 2022 Feb 18;14(2):149. doi: 10.3390/toxins14020149. Toxins (Basel). 2022. PMID: 35202176 Free PMC article.
-
Protein-macrocycle polymorphism: crystal form IV of the Ralstonia solanacearum lectin-sulfonato-calix[8]arene complex.Acta Crystallogr D Struct Biol. 2023 Jul 1;79(Pt 7):624-631. doi: 10.1107/S2059798323003832. Epub 2023 Jun 14. Acta Crystallogr D Struct Biol. 2023. PMID: 37314405 Free PMC article.
References
-
- Hekmat D, Hebel D, Weuster-Botz D. Chem. Eng. & Tech. 2008;31:911–916.
-
- Pechenov S, Shenoy B, Yang MX, Basu SK, Margolin AL. J. Control. Rel. 2004;96:149–158. - PubMed
-
- Basu SK, Govardhan CP, Jung CW, Margolin AL. Expert Opn. Biol. Therapy. 2004;4:301–317. - PubMed
-
- Shire SJ, Shahrokh Z, Liu JJ. Pharm. Sci. 2004;93:1390–1402. - PubMed
-
- Lafont S, Veesler S, Astier JP, Boistelle RJ. Cryst. Growth. 1994;143:249–255. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
