Synthetic Studies of Glycosylphosphatidylinositol (GPI) Anchors and GPI-Anchored Peptides, Glycopeptides, and Proteins
- PMID: 24955081
- PMCID: PMC4063365
- DOI: 10.2174/1570179411310030003
Synthetic Studies of Glycosylphosphatidylinositol (GPI) Anchors and GPI-Anchored Peptides, Glycopeptides, and Proteins
Abstract
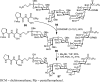
Glycosylphosphatidylinositol (GPI) anchorage of proteins and glycoproteins onto the cell surface is ubiquitous in eukaryotes, and GPI-anchored proteins and glycoproteins play an important role in many biological processes. To study GPI anchorage and explore the functions of GPIs and GPI-anchored proteins and glycoproteins, it is essential to have access to these molecules in homogeneous and structurally defined forms. This review is focused on the progress that our laboratory has made towards the chemical and chemoenzymatic synthesis of structurally defined GPI anchors and GPI-anchored peptides, glycopeptides, and proteins. Briefly, highly convergent strategies were developed for GPI synthesis and were employed to successfully synthesize a number of GPIs, including those carrying unsaturated lipids and other useful functionalities such as the azido and alkynyl groups. The latter enabled further site-specific modification of GPIs by click chemistry. GPI-linked peptides, glycopeptides, and proteins were prepared by regioselective chemical coupling of properly protected GPIs and peptides/glycopeptides or through site-specific ligation of synthetic GPIs and peptides/glycopeptides/proteins under the influence of sortase A. The investigation of interactions between GPI anchors and pore-forming bacterial toxins by means of synthetic GPI anchors and GPI analogs is also discussed.
Keywords: Carbohydrate; GPI; Glycolipid; Glycopeptide; Glycosylphosphatidylinositol; Peptide; Protein; Sortase.
Figures







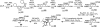
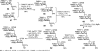





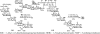



Similar articles
-
Chemical synthesis and functionalization of clickable glycosylphosphatidylinositol anchors.Chem Sci. 2011;2(12):2342-2352. doi: 10.1039/C1SC00440A. Chem Sci. 2011. PMID: 22163072 Free PMC article.
-
Chemoenzymatic synthesis of glycosylphosphatidylinositol-anchored glycopeptides.Chem Commun (Camb). 2010 Aug 21;46(31):5773-4. doi: 10.1039/c0cc00828a. Epub 2010 Jun 24. Chem Commun (Camb). 2010. PMID: 20574591 Free PMC article.
-
Recent research progress in glycosylphosphatidylinositol-anchored protein biosynthesis, chemical/chemoenzymatic synthesis, and interaction with the cell membrane.Curr Opin Chem Biol. 2024 Feb;78:102421. doi: 10.1016/j.cbpa.2023.102421. Epub 2024 Jan 4. Curr Opin Chem Biol. 2024. PMID: 38181647 Free PMC article. Review.
-
Sortase A-catalyzed transpeptidation of glycosylphosphatidylinositol derivatives for chemoenzymatic synthesis of GPI-anchored proteins.J Am Chem Soc. 2010 Feb 10;132(5):1567-71. doi: 10.1021/ja906611x. J Am Chem Soc. 2010. PMID: 20078124
-
Recent progress in synthetic and biological studies of GPI anchors and GPI-anchored proteins.Curr Opin Chem Biol. 2013 Dec;17(6):1006-13. doi: 10.1016/j.cbpa.2013.09.016. Epub 2013 Oct 12. Curr Opin Chem Biol. 2013. PMID: 24128440 Free PMC article. Review.
Cited by
-
(Patho)Physiology of Glycosylphosphatidylinositol-Anchored Proteins II: Intercellular Transfer of Matter (Inheritance?) That Matters.Biomolecules. 2023 Jun 15;13(6):994. doi: 10.3390/biom13060994. Biomolecules. 2023. PMID: 37371574 Free PMC article. Review.
-
Recombinant-Chemosynthetic Biosensors for Probing Cell Surface Signaling of Red Blood Cells and Other Cells.Chem Biomed Imaging. 2025 Jan 3;3(2):95-110. doi: 10.1021/cbmi.4c00067. eCollection 2025 Feb 24. Chem Biomed Imaging. 2025. PMID: 40018647 Free PMC article.
-
Stereospecific synthesis of phosphatidylglycerol using a cyanoethyl phosphoramidite precursor.Chem Phys Lipids. 2020 Sep;231:104933. doi: 10.1016/j.chemphyslip.2020.104933. Epub 2020 Jun 11. Chem Phys Lipids. 2020. PMID: 32533981 Free PMC article.
-
Labeling Cell Surface GPIs and GPI-Anchored Proteins through Metabolic Engineering with Artificial Inositol Derivatives.Angew Chem Int Ed Engl. 2015 Aug 10;54(33):9679-9682. doi: 10.1002/anie.201503814. Epub 2015 Jun 23. Angew Chem Int Ed Engl. 2015. PMID: 26102235 Free PMC article.
-
A synthetic cyclitol-nucleoside conjugate polyphosphate is a highly potent second messenger mimic.Chem Sci. 2019 May 2;10(20):5382-5390. doi: 10.1039/C9SC00445A. Epub 2019 Apr 23. Chem Sci. 2019. PMID: 31171961 Free PMC article.
References
-
- Ferguson MAJ, Williams AF. Cell-surface anchoring of proteins via glycosylphosphatidylinositol structure. Ann. Rev. Biochem. 1988;57:285–320. - PubMed
-
- Englund PT. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Ann. Rev. Biochem. 1993;62:121–138. - PubMed
-
- Eisenhaber B, Bork P, Eisenhaber F. Post-translational GPI lipid anchor modification of proteins in kingdoms of life: analysis of protein sequence data from complete genomes. Protein Eng. 2001;14:17–25. - PubMed
-
- Udenfriend S, Kodukula K. How glycosylphosphatidylinositol-anchored membrane protein are made. Ann. Rev. Biochem. 1995;64:563–591. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
