Safety of Intravenous Application of Mistletoe (Viscum album L.) Preparations in Oncology: An Observational Study
- PMID: 24955100
- PMCID: PMC4052504
- DOI: 10.1155/2014/236310
Safety of Intravenous Application of Mistletoe (Viscum album L.) Preparations in Oncology: An Observational Study
Abstract
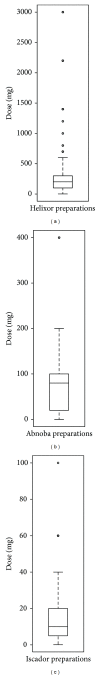
Background. Traditional mistletoe therapy in cancer patients involves subcutaneous applications of Viscum album L. preparations, with doses slowly increasing based on patient responses. Intravenous infusion of high doses may improve therapeutic outcomes and is becoming more common. Little is known about the safety of this "off-label" application of mistletoe. Methods. An observational study was performed within the Network Oncology. Treatment with intravenous mistletoe applications is described. The frequency of adverse drug reactions (ADRs) to intravenous mistletoe applications was calculated and compared to ADR data from a study on subcutaneous applications. Results. Of 475 cancer patients who received intravenous infusions of Helixor, Abnoba viscum, or Iscador mistletoe preparations, 22 patients (4.6%) reported 32 ADRs of mild (59.4%) or moderate severity (40.6%). No serious ADRs occurred. ADRs were more frequently reported to i.v. mistletoe administered alone (4.3%), versus prior to chemotherapy (1.6%). ADR frequency differed with respect to preparation type, with Iscador preparations showing a higher relative frequency, compared to Abnoba viscum and Helixor. Overall, patients were almost two times less likely to experience an ADR to intravenous compared to subcutaneous application of mistletoe. Conclusion. Intravenous mistletoe therapy was found to be safe and prospective studies for efficacy are recommended.
Figures
References
-
- Orange M, Fonseca M, Lace A, von Laue HB, Geider S. Durable tumour responses following primary high dose induction with mistletoe extracts: two case reports. European Journal of Integrative Medicine. 2010;2(2):63–69.
-
- Huber R, Barth H, Schmitt-Gräff A, Klein R. Hypereosinophilia induced by high-dose intratumoral and peritumoral mistletoe application to a patient with pancreatic carcinoma. Journal of Alternative and Complementary Medicine. 2000;6(4):305–310. - PubMed
-
- Schad F, Axtner J, Buchwald D, et al. Intratumoral mistletoe (Viscum album L) therapy in patients with unresectable pancreas carcinoma: a retrospective analysis. Integrative Cancer Therapies. 2013 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

