Enhanced plasmonic resonance energy transfer in mesoporous silica-encased gold nanorod for two-photon-activated photodynamic therapy
- PMID: 24955141
- PMCID: PMC4063978
- DOI: 10.7150/thno.8934
Enhanced plasmonic resonance energy transfer in mesoporous silica-encased gold nanorod for two-photon-activated photodynamic therapy
Abstract
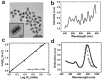
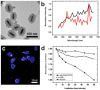
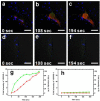
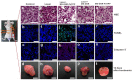
The unique optical properties of gold nanorods (GNRs) have recently drawn considerable interest from those working in in vivo biomolecular sensing and bioimaging. Especially appealing in these applications is the plasmon-enhanced photoluminescence of GNRs induced by two-photon excitation at infrared wavelengths, owing to the significant penetration depth of infrared light in tissue. Unfortunately, many studies have also shown that often the intensity of pulsed coherent irradiation of GNRs needed results in irreversible deformation of GNRs, greatly reducing their two-photon luminescence (TPL) emission intensity. In this work we report the design, synthesis, and evaluation of mesoporous silica-encased gold nanorods (MS-GNRs) that incorporate photosensitizers (PSs) for two-photon-activated photodynamic therapy (TPA-PDT). The PSs, doped into the nano-channels of the mesoporous silica shell, can be efficiently excited via intra-particle plasmonic resonance energy transfer from the encased two-photon excited gold nanorod and further generates cytotoxic singlet oxygen for cancer eradication. In addition, due to the mechanical support provided by encapsulating mesoporous silica matrix against thermal deformation, the two-photon luminescence stability of GNRs was significantly improved; after 100 seconds of 800 nm repetitive laser pulse with the 30 times higher than average power for imaging acquisition, MS-GNR luminescence intensity exhibited ~260% better resistance to deformation than that of the uncoated gold nanorods. These results strongly suggest that MS-GNRs with embedded PSs might provide a promising photodynamic therapy for the treatment of deeply situated cancers via plasmonic resonance energy transfer.
Keywords: Gold nanorods; photodynamic therapy; plasmonic resonance energy transfer; surface plasmon resonance; two-photon luminescence..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Enhancing fluorescence of quantum dots by silica-coated gold nanorods under one- and two-photon excitation.Opt Express. 2010 May 24;18(11):11335-46. doi: 10.1364/OE.18.011335. Opt Express. 2010. PMID: 20588995
-
Gold nanorod enhanced conjugated polymer/photosensitizer composite nanoparticles for simultaneous two-photon excitation fluorescence imaging and photodynamic therapy.Nanoscale. 2019 Nov 7;11(41):19551-19560. doi: 10.1039/c9nr05488j. Epub 2019 Oct 3. Nanoscale. 2019. PMID: 31578535
-
Effective Distribution of Gold Nanorods in Ordered Thick Mesoporous Silica: A Choice of Noninvasive Theranostics.ACS Appl Mater Interfaces. 2023 Oct 11;15(40):47615-47627. doi: 10.1021/acsami.3c06108. Epub 2023 Oct 2. ACS Appl Mater Interfaces. 2023. PMID: 37782885
-
Mesoporous Silica-Based Nanoparticles for Light-Actuated Biomedical Applications via Near-Infrared Two-Photon Absorption.Enzymes. 2018;43:67-99. doi: 10.1016/bs.enz.2018.07.004. Epub 2018 Sep 14. Enzymes. 2018. PMID: 30244809 Review.
-
Two-photon excitation nanoparticles for photodynamic therapy.Chem Soc Rev. 2016 Dec 21;45(24):6725-6741. doi: 10.1039/c6cs00442c. Epub 2016 Oct 5. Chem Soc Rev. 2016. PMID: 27711672 Review.
Cited by
-
Choice of Nanoparticles for Theranostics Engineering: Surface Coating to Nanovalves Approach.Nanotheranostics. 2024 Jan 1;8(1):12-32. doi: 10.7150/ntno.89768. eCollection 2024. Nanotheranostics. 2024. PMID: 38164501 Free PMC article. Review.
-
Exploring Low-Power Single-Pulsed Laser-Triggered Two-Photon Photodynamic/Photothermal Combination Therapy Using a Gold Nanostar/Graphene Quantum Dot Nanohybrid.ACS Appl Mater Interfaces. 2023 May 3;15(17):20811-20821. doi: 10.1021/acsami.3c03578. Epub 2023 Apr 21. ACS Appl Mater Interfaces. 2023. PMID: 37083346 Free PMC article.
-
Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy.Chem Soc Rev. 2016 Nov 21;45(23):6597-6626. doi: 10.1039/c6cs00271d. Chem Soc Rev. 2016. PMID: 27722328 Free PMC article. Review.
-
Enhanced Photodynamic Therapy: A Review of Combined Energy Sources.Cells. 2022 Dec 10;11(24):3995. doi: 10.3390/cells11243995. Cells. 2022. PMID: 36552759 Free PMC article. Review.
-
Helicobacter Pylori-Induced Gastric Infections: From Pathogenesis to Novel Therapeutic Approaches Using Silver Nanoparticles.Pharmaceutics. 2022 Jul 14;14(7):1463. doi: 10.3390/pharmaceutics14071463. Pharmaceutics. 2022. PMID: 35890358 Free PMC article. Review.
References
-
- Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2012;64:190–199. - PubMed
-
- Liu CL, Wu HT, Hsiao YH, Lai CW, Shih CW, Peng YK, Tang KC, Chang H W, Chien YC, Hsiao JK. Insulin-directed synthesis of fluorescent gold nanoclusters: preservation of insulin bioactivity and versatility in cell imaging. Angew Chem Int Edit. 2011;50:7056–7060. - PubMed
-
- Qian J, Zhan QQ, Li X, He SL. A study of mesoporous silica-encapsulated gold nanorods as enhanced light scattering probes for cancer cell imaging. Nanotechnology. 2010;21(5):055704–055712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

