Incorporation of Sulfated Hyaluronic Acid Macromers into Degradable Hydrogel Scaffolds for Sustained Molecule Delivery
- PMID: 24955239
- PMCID: PMC4060972
- DOI: 10.1039/C3BM60227C
Incorporation of Sulfated Hyaluronic Acid Macromers into Degradable Hydrogel Scaffolds for Sustained Molecule Delivery
Abstract
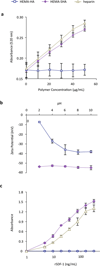
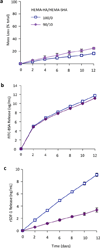
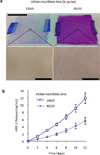
Synthetically sulfated hyaluronic acid (HA) has been shown to bind proteins with high affinity through electrostatic interactions. While HA-based hydrogels have been used widely in recent years for drug delivery and tissue engineering applications, incorporation of sulfated HA into these networks to attenuate the release of proteins has yet to be explored. Here, we developed sulfated and methacrylate-modified HA macromers and incorporated them into HA hydrogels through free radical-initiated crosslinking. The sulfated HA macromers bound a heparin-binding protein (i.e., stromal cell-derived factor 1-α, SDF-1α) with an affinity comparable to heparin and did not alter the gelation behavior or network mechanics when copolymerized into hydrogels at low concentrations. Further, these macromers were incorporated into electrospun nanofibrous hydrogels to introduce sulfate groups into macroporous scaffolds. Once incorporated into either uniform or fibrous HA hydrogels, the sulfated HA macromers significantly slowed encapsulated SDF-1α release over 12 days. Thus, these macromers provide a useful way to introduce heparin-binding features into radically-crosslinked hydrogels to alter protein interactions for a range of applications.
Figures


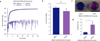


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

