The Redox-A3 Reaction
- PMID: 24955245
- PMCID: PMC4061755
- DOI: 10.1039/C4QO00022F
The Redox-A3 Reaction
Abstract
This Highlight details the recent emergence of a new type of A3 reaction (three-component condensation of an amine, an aldehyde and an alkyne). In contrast to the classic A3 coupling process, the redox-A3 reaction incorporates an iminium isomerization step and leads to amine α-alkynylation. The overall transformation is redox-neutral by virtue of a combined reductive N-alkylation/oxidative C-H bond functionalization.
Figures
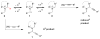
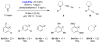




References
-
-
Selected reviews on the A3 reaction: Wei C, Li Z, Li CJ. Synlett. 2004:1472.Zani L, Bolm C. Chem Commun. 2006:4263.Peshkov VA, Pereshivko OP, Van der Eycken EV. Chem Soc Rev. 2012;41:3790.
-
-
-
Selected recent reviews on amine α-functionalization: Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Chem Eur J. 2012;18:10092.Peng B, Maulide N. Chem Eur J. 2013;19:13274.Qin Y, Lv J, Luo S. Tetrahedron Lett. 2014;55:551.Zheng C, You SL. RSC Adv. 2014;4:6173.
-
-
-
Selected recent reviews on the CDC reaction: Yeung CS, Dong VM. Chem Rev. 2011;111:1215.Girard SA, Knauber T, Li CJ. Angew Chem Int Ed. 2014;53:74.
-
-
- Bi HP, Teng Q, Guan M, Chen WW, Liang YM, Yao X, Li CJ. J Org Chem. 2010;75:783. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
