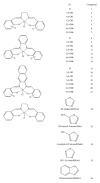
Structure-activity relationship for Fe(III)-salen-like complexes as potent anticancer agents
- PMID: 24955417
- PMCID: PMC3997896
- DOI: 10.1155/2014/745649
Structure-activity relationship for Fe(III)-salen-like complexes as potent anticancer agents
Abstract
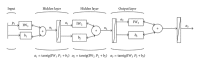
Quantitative structure activity relationship (QSAR) for the anticancer activity of Fe(III)-salen and salen-like complexes was studied. The methods of density function theory (B3LYP/LANL2DZ) were used to optimize the structures. A pool of descriptors was calculated: 1497 theoretical descriptors and quantum-chemical parameters, shielding NMR, and electronic descriptors. The study of structure and activity relationship was performed with multiple linear regression (MLR) and artificial neural network (ANN). In nonlinear method, the adaptive neuro-fuzzy inference system (ANFIS) was applied in order to choose the most effective descriptors. The ANN-ANFIS model with high statistical significance (R (2) train = 0.99, RMSE = 0.138, and Q (2) LOO = 0.82) has better capability to predict the anticancer activity of the new compounds series of this family. Based on this study, anticancer activity of this compound is mainly dependent on the geometrical parameters, position, and the nature of the substituent of salen ligand.
Figures
Similar articles
-
Adaptive neuro-fuzzy inference system (ANFIS): a new approach to predictive modeling in QSAR applications: a study of neuro-fuzzy modeling of PCP-based NMDA receptor antagonists.Bioorg Med Chem. 2007 Jun 15;15(12):4265-82. doi: 10.1016/j.bmc.2007.03.065. Epub 2007 Mar 24. Bioorg Med Chem. 2007. PMID: 17434739
-
Inhibition activity prediction for a dataset of candidates' drug by combining fuzzy logic with MLR/ANN QSAR models.Chem Biol Drug Des. 2019 Jun;93(6):1139-1157. doi: 10.1111/cbdd.13511. Chem Biol Drug Des. 2019. PMID: 31343121
-
Comparative study of artificial neural network (ANN), adaptive neuro-fuzzy inference system (ANFIS) and multiple linear regression (MLR) for modeling of Cu (II) adsorption from aqueous solution using biochar derived from rambutan (Nephelium lappaceum) peel.Environ Monit Assess. 2020 Jun 17;192(7):439. doi: 10.1007/s10661-020-08268-4. Environ Monit Assess. 2020. PMID: 32556670
-
QSAR based docking studies of marine algal anticancer compounds as inhibitors of protein kinase B (PKBβ).Eur J Pharm Sci. 2015 Aug 30;76:110-8. doi: 10.1016/j.ejps.2015.04.026. Epub 2015 Apr 29. Eur J Pharm Sci. 2015. PMID: 25936945
-
QSAR Studies and Structure Property/Activity Relationships Applied in Pyrazine Derivatives as Antiproliferative Agents Against the BGC823.Acta Chim Slov. 2021 Dec 15;68(4):882-895. doi: 10.17344/acsi.2021.6875. Acta Chim Slov. 2021. PMID: 34918764
Cited by
-
A metal-free salalen ligand with anti-tumor and synergistic activity in resistant leukemia and solid tumor cells via mitochondrial pathway.J Cancer Res Clin Oncol. 2021 Sep;147(9):2591-2607. doi: 10.1007/s00432-021-03679-3. Epub 2021 Jul 2. J Cancer Res Clin Oncol. 2021. PMID: 34213662 Free PMC article.
-
N-Heterocyclic Carbene Iron Complexes as Anticancer Agents: In Vitro and In Vivo Biological Studies.Molecules. 2021 Sep 12;26(18):5535. doi: 10.3390/molecules26185535. Molecules. 2021. PMID: 34577006 Free PMC article.
References
-
- Bravo-Gómez ME, García-Ramos JC, Gracia-Mora I, Ruiz-Azuara L. Antiproliferative activity and QSAR study of copper(II) mixed chelate [Cu(N-N)(acetylacetonato)]NO3 and [Cu(N-N)(glycinato)]NO3 complexes. Journal of Inorganic Biochemistry. 2009;103(2):299–309. - PubMed
-
- Ansari KI, Kasiri S, Grant JD, Mandal SS. Fe(III)-salen and salphen complexes induce caspase activation and apoptosis in human cells. Journal of Biomolecular Screening. 2010;16:26–35. - PubMed
-
- Khan N-UH, Pandya N, Kureshy RI, et al. Synthesis, characterization, DNA binding and cleavage studies of chiral Ru(II) salen complexes. Spectrochimica Acta A: Molecular and Biomolecular Spectroscopy. 2009;74(1):113–119. - PubMed
-
- Zhang N, Fan Yh, Zhang Z. Syntheses, crystal structures and anticancer activities of three novel transition metal complexes with Schiff base derived from 2-acetylpyridine and l-tryptophan. Inorganic Chemistry Communications. 2012;22:68–72.
-
- Ali I, Wani WA, Saleem K, Hseih MF. Design and synthesis of thalidomide based dithiocarbamate Cu(II), Ni(II) and Ru(III) complexes as anticancer agents. Polyhedron. 2013;56:134–143.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources