Telemedicine approaches to evaluating acute-phase retinopathy of prematurity: study design
- PMID: 24955738
- PMCID: PMC4861056
- DOI: 10.3109/09286586.2014.926940
Telemedicine approaches to evaluating acute-phase retinopathy of prematurity: study design
Abstract
Purpose: Detecting sight-threatening retinopathy of prematurity (ROP) relies on a diagnostic examination (DE) performed by an experienced ophthalmologist. An alternative may be a telemedicine system where retinal images of at-risk infants are graded by readers to determine features of ROP indicating the need for a DE.
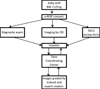
Methods: The multicenter Telemedicine Approaches to Evaluating Acute-phase ROP (e-ROP) Study is a cohort study of 2000 infants with birth weights <1251 g. At each visit, ophthalmologists perform DEs and non-physician imagers obtain iris and five retinal images with the disc positioned in the center, right, left, up and down. Images are uploaded to a secure server for grading by non-physician readers for the detection of plus disease, stage 3 ROP and/or zone I disease, any of which indicates "referral-warranted ROP" (RW-ROP). Images from all infants with RW-ROP and a random sample of infants without RW-ROP (based on DEs) are selected for grading. Gradings are compared to DEs to determine the validity and evaluate reliability, feasibility, safety, and cost-effectiveness of the telemedicine system.
Results: e-ROP is conducted in 12 Clinical Centers in the US and Canada with Study Headquarters, the Data Coordinating Center and the Image Reading Center in Philadelphia and the ROP Data Center in Oklahoma City. A total of 27 study center coordinators, 34 ophthalmologists, 26 imagers, and 4 readers have been certified. All study data are submitted using a secure web-based system.
Conclusion: The design and findings of this study will be useful to conduct other ROP studies or evaluate telemedicine for other diseases.
Trial registration: ClinicalTrials.gov NCT01264276.
Keywords: Childhood blindness; prematurity; retinopathy of prematurity; telemedicine.
Conflict of interest statement
None of the authors have any proprietary interests or conflicts of interest related to this submission.
Figures







References
-
- Gilbert C, Fielder A, Gordillo L, Quinn G, Semiglia R, Visintin P, Zin A on behalf of the International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate and high levels of development: Implications for screening programs. Pediatrics. 2005;115:e518–e525. - PubMed
-
- Gilbert C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Human Development. 2008;84:77–82. - PubMed
-
- Castillo-Riquelme MC, Lord J, Moseley MJ, Fielder AR, Haines L. Cost-effectiveness of digital photographic screening for retinopathy of prematurity in the United Kingdom. Int J Technol Assess Health Care. 2004;20(2):201–213. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
