TRAF6 inhibition rescues dexamethasone-induced muscle atrophy
- PMID: 24955790
- PMCID: PMC4100203
- DOI: 10.3390/ijms150611126
TRAF6 inhibition rescues dexamethasone-induced muscle atrophy
Abstract
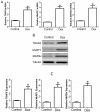
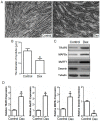
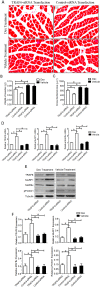
Tumor necrosis factor receptor-associated factor 6 (TRAF6), a unique E3 ubiquitin ligase and adaptor protein, is involved in activation of various signaling cascades. Recent studies identify TRAF6 as one of the novel regulators of skeletal muscle atrophy. The role of TRAF6 in glucocorticoid-induced muscle atrophy, however, remains to be elucidated. In this study, we show that TRAF6 and its downstream signaling molecules, muscle atrophy F-box (MAFBx) and muscle ring finger 1 (MuRF1), were all upregulated in dexamethasone-induced atrophy of mouse C2C12 myotubes or mouse tibialis anterior (TA) muscle. To further investigate the role of TRAF6 in dexamethasone-induced muscle atrophy, TRAF6-siRNA was used to transfect cultured C2C12 myotubes or was injected into the TA muscle of mice respectively, and we note that TRAF6 knockdown attenuated dexamethasone-induced muscle atrophy in vitro and in vivo, and concomitantly decreased the expression of MuRF1 and MAFBx. Our findings suggest that a decreased expression of TRAF6 could rescue dexamethasone-induced skeletal muscle atrophy through, at least in part, regulation of the expression of MAFBx and MuRF1.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

