The myeloid-binding peptide adenoviral vector enables multi-organ vascular endothelial gene targeting
- PMID: 24955893
- PMCID: PMC4117817
- DOI: 10.1038/labinvest.2014.78
The myeloid-binding peptide adenoviral vector enables multi-organ vascular endothelial gene targeting
Abstract
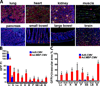
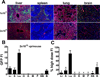
Vascular endothelial cells (ECs) are ideal gene therapy targets as they provide widespread tissue access and are the first contact surfaces following intravenous vector administration. Human recombinant adenovirus serotype 5 (Ad5) is the most frequently used gene transfer system because of its appreciable transgene payload capacity and lack of somatic mutation risk. However, standard Ad5 vectors predominantly transduce liver but not the vasculature following intravenous administration. We recently developed an Ad5 vector with a myeloid cell-binding peptide (MBP) incorporated into the knob-deleted, T4 fibritin chimeric fiber (Ad.MBP). This vector was shown to transduce pulmonary ECs presumably via a vector handoff mechanism. Here we tested the body-wide tropism of the Ad.MBP vector, its myeloid cell necessity, and vector-EC expression dose response. Using comprehensive multi-organ co-immunofluorescence analysis, we discovered that Ad.MBP produced widespread EC transduction in the lung, heart, kidney, skeletal muscle, pancreas, small bowel, and brain. Surprisingly, Ad.MBP retained hepatocyte tropism albeit at a reduced frequency compared with the standard Ad5. While binding specifically to myeloid cells ex vivo, multi-organ Ad.MBP expression was not dependent on circulating monocytes or macrophages. Ad.MBP dose de-escalation maintained full lung-targeting capacity but drastically reduced transgene expression in other organs. Swapping the EC-specific ROBO4 for the CMV promoter/enhancer abrogated hepatocyte expression but also reduced gene expression in other organs. Collectively, our multilevel targeting strategy could enable therapeutic biological production in previously inaccessible organs that pertain to the most debilitating or lethal human diseases.
Conflict of interest statement
All authors declare no financial conflict of interest.
Figures



References
-
- Nicol CG, Denby L, Lopez-Franco O, et al. Use of in vivo phage display to engineer novel adenoviruses for targeted delivery to the cardiac vasculature. FEBS letters. 2009;583(12):2100–2107. - PubMed
-
- Bradshaw AC, Baker AH. Gene therapy for cardiovascular disease: perspectives and potential. Vascular pharmacology. 2013;58(3):174–181. - PubMed
-
- Muro S, Koval M, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Current vascular pharmacology. 2004;2(3):281–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

