Preparation of laponite bioceramics for potential bone tissue engineering applications
- PMID: 24955961
- PMCID: PMC4067276
- DOI: 10.1371/journal.pone.0099585
Preparation of laponite bioceramics for potential bone tissue engineering applications
Abstract
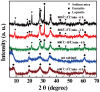
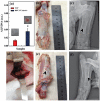
We report a facile approach to preparing laponite (LAP) bioceramics via sintering LAP powder compacts for bone tissue engineering applications. The sintering behavior and mechanical properties of LAP compacts under different temperatures, heating rates, and soaking times were investigated. We show that LAP bioceramic with a smooth and porous surface can be formed at 800°C with a heating rate of 5°C/h for 6 h under air. The formed LAP bioceramic was systematically characterized via different methods. Our results reveal that the LAP bioceramic possesses an excellent surface hydrophilicity and serum absorption capacity, and good cytocompatibility and hemocompatibility as demonstrated by resazurin reduction assay of rat mesenchymal stem cells (rMSCs) and hemolytic assay of pig red blood cells, respectively. The potential bone tissue engineering applicability of LAP bioceramic was explored by studying the surface mineralization behavior via soaking in simulated body fluid (SBF), as well as the surface cellular response of rMSCs. Our results suggest that LAP bioceramic is able to induce hydroxyapatite deposition on its surface when soaked in SBF and rMSCs can proliferate well on the LAP bioceramic surface. Most strikingly, alkaline phosphatase activity together with alizarin red staining results reveal that the produced LAP bioceramic is able to induce osteoblast differentiation of rMSCs in growth medium without any inducing factors. Finally, in vivo animal implantation, acute systemic toxicity test and hematoxylin and eosin (H&E)-staining data demonstrate that the prepared LAP bioceramic displays an excellent biosafety and is able to heal the bone defect. Findings from this study suggest that the developed LAP bioceramic holds a great promise for treating bone defects in bone tissue engineering.
Conflict of interest statement
Figures







Similar articles
-
Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells.Biomaterials. 2014 Oct;35(30):8514-27. doi: 10.1016/j.biomaterials.2014.06.028. Epub 2014 Jul 4. Biomaterials. 2014. PMID: 25002263
-
High temperature CaSiO3-Ca3(PO4)2 ceramic promotes osteogenic differentiation in adult human mesenchymal stem cells.Mater Sci Eng C Mater Biol Appl. 2020 Feb;107:110355. doi: 10.1016/j.msec.2019.110355. Epub 2019 Oct 24. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31761182
-
Decellularized extracellular matrix coupled with polycaprolactone/laponite to construct a biomimetic barrier membrane for bone defect repair.Int J Biol Macromol. 2024 Sep;276(Pt 1):133775. doi: 10.1016/j.ijbiomac.2024.133775. Epub 2024 Jul 8. Int J Biol Macromol. 2024. PMID: 38986979
-
Fabrication Strategies for Bioceramic Scaffolds in Bone Tissue Engineering with Generative Design Applications.Biomimetics (Basel). 2024 Jul 5;9(7):409. doi: 10.3390/biomimetics9070409. Biomimetics (Basel). 2024. PMID: 39056850 Free PMC article. Review.
-
Three-Dimensional Printing Methods for Bioceramic-Based Scaffold Fabrication for Craniomaxillofacial Bone Tissue Engineering.J Funct Biomater. 2024 Mar 1;15(3):60. doi: 10.3390/jfb15030060. J Funct Biomater. 2024. PMID: 38535253 Free PMC article. Review.
Cited by
-
Emerging 2D Nanomaterials for Biomedical Applications.Mater Today (Kidlington). 2021 Nov;50:276-302. doi: 10.1016/j.mattod.2021.04.020. Epub 2021 Jun 17. Mater Today (Kidlington). 2021. PMID: 34970073 Free PMC article.
-
LAPONITE® nanorods regulating degradability, acidic-alkaline microenvironment, apatite mineralization and MC3T3-E1 cells responses to poly(butylene succinate) based bio-nanocomposite scaffolds.RSC Adv. 2018 Mar 19;8(20):10794-10805. doi: 10.1039/c7ra13452e. eCollection 2018 Mar 16. RSC Adv. 2018. PMID: 35541558 Free PMC article.
-
Autologous protein-based scaffold composed of platelet lysate and aminated hyaluronic acid.J Mater Sci Mater Med. 2019 Nov 25;30(12):127. doi: 10.1007/s10856-019-6334-7. J Mater Sci Mater Med. 2019. PMID: 31768643
-
Alkaline shear-thinning micro-nanocomposite hydrogels initiate endogenous TGFβ signaling for in situ bone regeneration.NPJ Regen Med. 2023 Oct 13;8(1):56. doi: 10.1038/s41536-023-00333-z. NPJ Regen Med. 2023. PMID: 37833374 Free PMC article.
-
Laponite/amoxicillin-functionalized PLA nanofibrous as osteoinductive and antibacterial scaffolds.Sci Rep. 2022 Apr 21;12(1):6583. doi: 10.1038/s41598-022-10595-0. Sci Rep. 2022. PMID: 35449188 Free PMC article.
References
-
- Murugan R, Ramakrishna S (2004) Bioresorbable composite bone paste using polysaccharide based nano hydroxyapatite. Biomaterials 25: 3829–3835. - PubMed
-
- Zaidman N, Bosnakovski D (2012) Advancing with ceramic biocomposites for bone graft implants. Recent Patents on Regenerative Medicine 2: 65–72.
-
- Swetha M, Sahithi K, Moorthi A, Srinivasan N, Ramasamy K, et al. (2010) Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int J Biol Macromol 47: 1–4. - PubMed
-
- Barbieri D, Yuan H, Luo X, Farè S, Grijpma DW, et al. (2013) Influence of polymer molecular weight in osteoinductive composites for bone tissue regeneration. Acta Biomater 9. - PubMed
-
- Cao Z, Wen J, Yao J, Chen X, Ni Y, et al. (2013) Facile fabrication of the porous 3-dimensional regenerated silk fibroin scaffolds. Mater Sci Eng C-Mater Biol Appl 33: 3522–3529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

