Impact of nitrogen sources on gene expression and toxin production in the diazotroph Cylindrospermopsis raciborskii CS-505 and non-diazotroph Raphidiopsis brookii D9
- PMID: 24956074
- PMCID: PMC4073136
- DOI: 10.3390/toxins6061896
Impact of nitrogen sources on gene expression and toxin production in the diazotroph Cylindrospermopsis raciborskii CS-505 and non-diazotroph Raphidiopsis brookii D9
Abstract
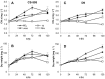
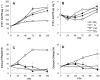
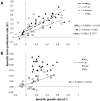
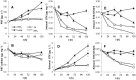
Different environmental nitrogen sources play selective roles in the development of cyanobacterial blooms and noxious effects are often exacerbated when toxic cyanobacteria are dominant. Cylindrospermopsis raciborskii CS-505 (heterocystous, nitrogen fixing) and Raphidiopsis brookii D9 (non-N₂ fixing) produce the nitrogenous toxins cylindrospermopsin (CYN) and paralytic shellfish toxins (PSTs), respectively. These toxin groups are biosynthesized constitutively by two independent putative gene clusters, whose flanking genes are target for nitrogen (N) regulation. It is not yet known how or if toxin biosynthetic genes are regulated, particularly by N-source dependency. Here we show that binding boxes for NtcA, the master regulator of N metabolism, are located within both gene clusters as potential regulators of toxin biosynthesis. Quantification of intra- and extracellular toxin content in cultures at early stages of growth under nitrate, ammonium, urea and N-free media showed that N-sources influence neither CYN nor PST production. However, CYN and PST profiles were altered under N-free medium resulting in a decrease in the predicted precursor toxins (doCYN and STX, respectively). Reduced STX amounts were also observed under growth in ammonium. Quantification of toxin biosynthesis and transport gene transcripts revealed a constitutive transcription under all tested N-sources. Our data support the hypothesis that PSTs and CYN are constitutive metabolites whose biosynthesis is correlated to cyanobacterial growth rather than directly to specific environmental conditions. Overall, the constant biosynthesis of toxins and expression of the putative toxin-biosynthesis genes supports the usage of qPCR probes in water quality monitoring of toxic cyanobacteria.
Figures
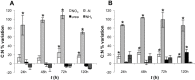
Similar articles
-
Comparative genomics of Cylindrospermopsis raciborskii strains with differential toxicities.BMC Genomics. 2014 Jan 29;15:83. doi: 10.1186/1471-2164-15-83. BMC Genomics. 2014. PMID: 24476316 Free PMC article.
-
Transcriptional regulation of the cylindrospermopsin biosynthesis (cyr) gene cluster in Raphidiopsis raciborskii AWT205.Harmful Algae. 2025 Feb;142:102783. doi: 10.1016/j.hal.2024.102783. Epub 2024 Dec 4. Harmful Algae. 2025. PMID: 39947847
-
Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis.Appl Environ Microbiol. 2008 Feb;74(3):716-22. doi: 10.1128/AEM.01988-07. Epub 2007 Dec 7. Appl Environ Microbiol. 2008. PMID: 18065631 Free PMC article.
-
Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: A molecular overview.J Proteomics. 2016 Mar 1;135:132-140. doi: 10.1016/j.jprot.2015.08.008. Epub 2015 Aug 25. J Proteomics. 2016. PMID: 26316331 Review.
-
In search of environmental role of cylindrospermopsin: a review on global distribution and ecology of its producers.Water Res. 2014 Dec 1;66:320-337. doi: 10.1016/j.watres.2014.08.029. Epub 2014 Sep 3. Water Res. 2014. PMID: 25222334 Review.
Cited by
-
Cyanotoxins, biosynthetic gene clusters, and factors modulating cyanotoxin biosynthesis.World J Microbiol Biotechnol. 2023 Jul 3;39(9):241. doi: 10.1007/s11274-023-03652-x. World J Microbiol Biotechnol. 2023. PMID: 37394567 Review.
-
Nitrogen and phosphorus significantly alter growth, nitrogen fixation, anatoxin-a content, and the transcriptome of the bloom-forming cyanobacterium, Dolichospermum.Front Microbiol. 2022 Sep 7;13:955032. doi: 10.3389/fmicb.2022.955032. eCollection 2022. Front Microbiol. 2022. PMID: 36160233 Free PMC article.
-
Moving towards adaptive management of cyanotoxin-impaired water bodies.Microb Biotechnol. 2016 Sep;9(5):641-51. doi: 10.1111/1751-7915.12383. Epub 2016 Jul 15. Microb Biotechnol. 2016. PMID: 27418325 Free PMC article. Review.
-
Dynamics of the toxin cylindrospermopsin and the cyanobacterium Chrysosporum (Aphanizomenon) ovalisporum in a Mediterranean eutrophic reservoir.Toxins (Basel). 2014 Oct 28;6(11):3041-57. doi: 10.3390/toxins6113041. Toxins (Basel). 2014. PMID: 25354130 Free PMC article.
-
Health and Environmental Impacts of Cyanobacteria and Cyanotoxins from Freshwater to Seawater.Toxins (Basel). 2025 Mar 7;17(3):126. doi: 10.3390/toxins17030126. Toxins (Basel). 2025. PMID: 40137899 Free PMC article. Review.
References
-
- Carmichael W.W. The cyanotoxins. Adv. Bot. Res. 1997;27:211–256. doi: 10.1016/S0065-2296(08)60282-7. - DOI
-
- Sivonen K., Jones G. Cyanobacterial toxins. In: Chrorus I., Bartam J., editors. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences,Monitoring and Management. E & FN Spon; London, UK: 1999. pp. 41–111.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

