S100A8/A9 mRNA induction in an ex vivo model of endotoxin tolerance: roles of IL-10 and IFNγ
- PMID: 24956170
- PMCID: PMC4067416
- DOI: 10.1371/journal.pone.0100909
S100A8/A9 mRNA induction in an ex vivo model of endotoxin tolerance: roles of IL-10 and IFNγ
Abstract
Objectives: Septic syndromes are the leading cause of death in intensive care units. They are characterized by the development of immune dysfunctions such as endotoxin tolerance (ET), whose intensity and duration are associated with increased risk of nosocomial infections and mortality. Alarmins S100A8 and S100A9 have been shown to be increased after septic shock. Importantly, a delayed S100A9 mRNA increase predicts hospital-acquired infection in patients. The aim of this study was to investigate the regulation of S100A8 and S100A9 mRNA expression in an ex vivo model of ET.
Subjects and measurements: ET was reproduced ex vivo by priming healthy peripheral blood mononuclear cells (number of donors = 9 to 10) with low-dose endotoxin (2 ng/ml) before stimulation with high dose endotoxin (100 ng/ml). S100A8 and S100A9 mRNA levels were measured by quantitative real-time polymerase chain reactions.
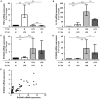
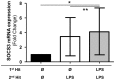
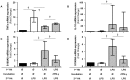
Main results: ET was established by observing decreased TNFα and increased IL-10 transcriptomic responses to two subsequent endotoxin challenges. Interestingly, ET was associated with increased S100A8 and S100A9 mRNA expression ex vivo. We showed that IL-10 played a role in this process, since S100A8 and S100A9 mRNA increases were significantly abrogated by IL-10 blockade in the model. Conversely, treatment with rIFN-γ, a pro-inflammatory and immunostimulating molecule known to block ET induction, was able to restore normal S100A8 and S100A9 mRNA in this model.
Conclusions: In this ex vivo model, we observed that S100A8 and S100A9 mRNA expression was significantly increased during ET. This reproduced ex vivo the observations we had previously made in septic shock patients. Interestingly, IL-10 blockade and rIFN-γ treatment partially abrogated S100A8/A9 mRNA increases in this model. Pending confirmation in larger, independent clinical studies, these preliminary results suggest that S100A8 and S100A9 mRNA levels might be used as surrogate markers of ET and as stratification tools for personalized immunotherapy in septic shock patients.
Conflict of interest statement
Figures


References
-
- Cavaillon J-M, Adrie C, Fitting C, Adib-Conquy M (2003) Endotoxin tolerance: is there a clinical relevance? J Endotoxin Res 9: 101–107. - PubMed
-
- Goyette J, Geczy CL (2011) Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids 41: 821–842. - PubMed
-
- Sroussi HY, Berline J, Palefsky JM (2007) Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol 81: 818–824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

