Integration of existing systematic reviews into new reviews: identification of guidance needs
- PMID: 24956937
- PMCID: PMC4066698
- DOI: 10.1186/2046-4053-3-60
Integration of existing systematic reviews into new reviews: identification of guidance needs
Abstract
Background: An exponential increase in the number of systematic reviews published, and constrained resources for new reviews, means that there is an urgent need for guidance on explicitly and transparently integrating existing reviews into new systematic reviews. The objectives of this paper are: 1) to identify areas where existing guidance may be adopted or adapted, and 2) to suggest areas for future guidance development.
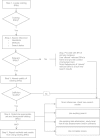
Methods: We searched documents and websites from healthcare focused systematic review organizations to identify and, where available, to summarize relevant guidance on the use of existing systematic reviews. We conducted informational interviews with members of Evidence-based Practice Centers (EPCs) to gather experiences in integrating existing systematic reviews, including common issues and challenges, as well as potential solutions.
Results: There was consensus among systematic review organizations and the EPCs about some aspects of incorporating existing systematic reviews into new reviews. Current guidance may be used in assessing the relevance of prior reviews and in scanning references of prior reviews to identify studies for a new review. However, areas of challenge remain. Areas in need of guidance include how to synthesize, grade the strength of, and present bodies of evidence composed of primary studies and existing systematic reviews. For instance, empiric evidence is needed regarding how to quality check data abstraction and when and how to use study-level risk of bias assessments from prior reviews.
Conclusions: There remain areas of uncertainty for how to integrate existing systematic reviews into new reviews. Methods research and consensus processes among systematic review organizations are needed to develop guidance to address these challenges.
Figures
References
-
- Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. 2012;10:1–199.
-
- Fordis M. Evidence-based Practice Centers IV Inaugural Meeting: December 12, 2012. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Observations in Translating CERs: Informing Report and Translation Product Development, Example 2.1; pp. 43–44.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources