Correlation of Gas Permeability in a Metal-Organic Framework MIL-101(Cr)-Polysulfone Mixed-Matrix Membrane with Free Volume Measurements by Positron Annihilation Lifetime Spectroscopy (PALS)
- PMID: 24957061
- PMCID: PMC4021949
- DOI: 10.3390/membranes3040331
Correlation of Gas Permeability in a Metal-Organic Framework MIL-101(Cr)-Polysulfone Mixed-Matrix Membrane with Free Volume Measurements by Positron Annihilation Lifetime Spectroscopy (PALS)
Abstract
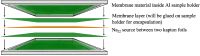
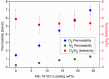
Hydrothermally stable particles of the metal-organic framework MIL-101(Cr) were incorporated into a polysulfone (PSF) matrix to produce mixed-matrix or composite membranes with excellent dispersion of MIL-101 particles and good adhesion within the polymer matrix. Pure gas (O2, N2, CO2 and CH4) permeation tests showed a significant increase of gas permeabilities of the mixed-matrix membranes without any loss in selectivity. Positron annihilation lifetime spectroscopy (PALS) indicated that the increased gas permeability is due to the free volume in the PSF polymer and the added large free volume inside the MIL-101 particles. The trend of the gas transport properties of the composite membranes could be reproduced by a Maxwell model.
Figures








Similar articles
-
Efficient CO2/CH4 Separation Using Polysulfone/NH2-MIL-125(Ti) Mixed Matrix Membranes.ACS Omega. 2025 Mar 18;10(12):11972-11979. doi: 10.1021/acsomega.4c09251. eCollection 2025 Apr 1. ACS Omega. 2025. PMID: 40191367 Free PMC article.
-
High performance MIL-101(Cr)@6FDA-mPD and MOF-199@6FDA-mPD mixed-matrix membranes for CO2/CH4 separation.Dalton Trans. 2020 Feb 11;49(6):1822-1829. doi: 10.1039/c9dt03222c. Dalton Trans. 2020. PMID: 31961353
-
High Selective Mixed Membranes Based on Mesoporous MCM-41 and MCM-41-NH2 Particles in a Polysulfone Matrix.Front Chem. 2019 Jun 17;7:332. doi: 10.3389/fchem.2019.00332. eCollection 2019. Front Chem. 2019. PMID: 31263688 Free PMC article.
-
Probing the Free Volume in Polymers by Means of Positron Annihilation Lifetime Spectroscopy.Polymers (Basel). 2023 Jul 23;15(14):3128. doi: 10.3390/polym15143128. Polymers (Basel). 2023. PMID: 37514518 Free PMC article. Review.
-
Application Progress of PALS in the Correlation of Structure and Properties for Graphene/Polymer Nanocomposites.Nanomaterials (Basel). 2022 Nov 24;12(23):4161. doi: 10.3390/nano12234161. Nanomaterials (Basel). 2022. PMID: 36500784 Free PMC article. Review.
Cited by
-
Challenges, Opportunities and Future Directions of Membrane Technology for Natural Gas Purification: A Critical Review.Membranes (Basel). 2022 Jun 23;12(7):646. doi: 10.3390/membranes12070646. Membranes (Basel). 2022. PMID: 35877848 Free PMC article. Review.
-
Revisiting Metal-Organic Frameworks Porosimetry by Positron Annihilation: Metal Ion States and Positronium Parameters.J Phys Chem Lett. 2024 May 2;15(17):4560-4567. doi: 10.1021/acs.jpclett.4c00762. Epub 2024 Apr 19. J Phys Chem Lett. 2024. PMID: 38638089 Free PMC article.
-
MOF@PEDOT Composite Films for Impedimetric Pesticide Sensors.Glob Chall. 2020 Jan 8;4(2):1900076. doi: 10.1002/gch2.201900076. eCollection 2020 Feb. Glob Chall. 2020. PMID: 32042446 Free PMC article.
-
Free volume and gas permeation in anthracene maleimide-based polymers of intrinsic microporosity.Membranes (Basel). 2015 May 28;5(2):214-27. doi: 10.3390/membranes5020214. Membranes (Basel). 2015. PMID: 26030881 Free PMC article.
-
Fe/Co-MOF Nanocatalysts: Greener Chemistry Approach for the Removal of Toxic Metals and Catalytic Applications.ACS Omega. 2022 Jun 27;7(27):23421-23444. doi: 10.1021/acsomega.2c01770. eCollection 2022 Jul 12. ACS Omega. 2022. PMID: 35847326 Free PMC article.
References
-
- Davis J.C., Valus R.J., Eshraghi R., Velikoff A.E. Facilitated transport membrane hybrid systems for olefin purification. Sep. Sci. Technol. 1993;28:463–476. doi: 10.1080/01496399308019500. - DOI
-
- Strathmann H. Membrane separation processes: Current relevance and future opportunities. AIChE J. 2001;47:1077–1087. doi: 10.1002/aic.690470514. - DOI
-
- Koros W.J., Mahajan R. Pushing the limits on possibilities for large scale gas separation: Which strategies? J. Membr. Sci. 2000;175:181–196. doi: 10.1016/S0376-7388(00)00418-X. - DOI
-
- Ohlrogge K., Stürken K. Membrane Technology. Wiley-VCH; Weinheim, Germany: 2001. The Separation of Organic Vapors from Gas Streams by Means of Membranes; pp. 69–94.
LinkOut - more resources
Full Text Sources
Other Literature Sources

