Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1
- PMID: 24957212
- PMCID: PMC4179131
- DOI: 10.14348/molcells.2014.0150
Regulation of wound healing and fibrosis by hypoxia and hypoxia-inducible factor-1
Abstract
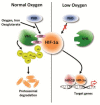
Wound healing is a complex multi-step process that requires spatial and temporal orchestration of cellular and non-cellular components. Hypoxia is one of the prominent microenvironmental factors in tissue injury and wound healing. Hypoxic responses, mainly mediated by a master transcription factor of oxygen homeostasis, hypoxia-inducible factor-1 (HIF-1), have been shown to be critically involved in virtually all processes of wound healing and remodeling. Yet, mechanisms underlying hypoxic regulation of wound healing are still poorly understood. Better understanding of how the wound healing process is regulated by the hypoxic microenvironment and HIF-1 signaling pathway will provide insight into the development of a novel therapeutic strategy for impaired wound healing conditions such as diabetic wound and fibrosis. In this review, we will discuss recent studies illuminating the roles of HIF-1 in physiologic and pathologic wound repair and further, the therapeutic potentials of HIF-1 stabilization or inhibition.
Keywords: fibrosis; hypoxia; hypoxia-inducible factor-1; oxygen; wound healing.
Figures
References
-
- Ahluwalia A, Tarnawski AS. Critical role of hypoxia sensor–HIF-1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem. 2012;19:90–97. - PubMed
-
- Andrikopoulou E, Zhang X, Sebastian R, Marti G, Liu L, Milner SM, Harmon JW. Current Insights into the role of HIF-1 in cutaneous wound healing. Curr Mol Med. 2011;11:218–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

