Volatile metabolites
- PMID: 24957243
- PMCID: PMC4012514
- DOI: 10.3390/metabo1010041
Volatile metabolites
Abstract
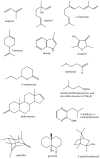
Volatile organic compounds (volatiles) comprise a chemically diverse class of low molecular weight organic compounds having an appreciable vapor pressure under ambient conditions. Volatiles produced by plants attract pollinators and seed dispersers, and provide defense against pests and pathogens. For insects, volatiles may act as pheromones directing social behavior or as cues for finding hosts or prey. For humans, volatiles are important as flavorants and as possible disease biomarkers. The marine environment is also a major source of halogenated and sulfur-containing volatiles which participate in the global cycling of these elements. While volatile analysis commonly measures a rather restricted set of analytes, the diverse and extreme physical properties of volatiles provide unique analytical challenges. Volatiles constitute only a small proportion of the total number of metabolites produced by living organisms, however, because of their roles as signaling molecules (semiochemicals) both within and between organisms, accurately measuring and determining the roles of these compounds is crucial to an integrated understanding of living systems. This review summarizes recent developments in volatile research from a metabolomics perspective with a focus on the role of recent technical innovation in developing new areas of volatile research and expanding the range of ecological interactions which may be mediated by volatile organic metabolites.
Figures

References
-
- Pichersky E., Gershenzon J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002;5:237–243. - PubMed
-
- Lundström J.N., Hummel T., Olsson M.J. Individual differences in sensitivity to the odor of 4,16-androstadien-3-one. Chem. Senses. 2003;28:643–650. - PubMed
-
- Heil M., Ton J. Long-distance signalling in plant defence. Trends Plant Sci. 2008;13:264–272. - PubMed
-
- Dicke M., van Loon J.J.A., Soler R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 2009;5:317–324. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

