CstF-64 supports pluripotency and regulates cell cycle progression in embryonic stem cells through histone 3' end processing
- PMID: 24957598
- PMCID: PMC4117776
- DOI: 10.1093/nar/gku551
CstF-64 supports pluripotency and regulates cell cycle progression in embryonic stem cells through histone 3' end processing
Abstract
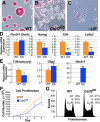
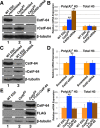
Embryonic stem cells (ESCs) exhibit a unique cell cycle with a shortened G1 phase that supports their pluripotency, while apparently buffering them against pro-differentiation stimuli. In ESCs, expression of replication-dependent histones is a main component of this abbreviated G1 phase, although the details of this mechanism are not well understood. Similarly, the role of 3' end processing in regulation of ESC pluripotency and cell cycle is poorly understood. To better understand these processes, we examined mouse ESCs that lack the 3' end-processing factor CstF-64. These ESCs display slower growth, loss of pluripotency and a lengthened G1 phase, correlating with increased polyadenylation of histone mRNAs. Interestingly, these ESCs also express the τCstF-64 paralog of CstF-64. However, τCstF-64 only partially compensates for lost CstF-64 function, despite being recruited to the histone mRNA 3' end-processing complex. Reduction of τCstF-64 in CstF-64-deficient ESCs results in even greater levels of histone mRNA polyadenylation, suggesting that both CstF-64 and τCstF-64 function to inhibit polyadenylation of histone mRNAs. These results suggest that CstF-64 plays a key role in modulating the cell cycle in ESCs while simultaneously controlling histone mRNA 3' end processing.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
3'-End processing of histone pre-mRNAs in Drosophila: U7 snRNP is associated with FLASH and polyadenylation factors.RNA. 2013 Dec;19(12):1726-44. doi: 10.1261/rna.040360.113. Epub 2013 Oct 21. RNA. 2013. PMID: 24145821 Free PMC article.
-
Interactions of CstF-64, CstF-77, and symplekin: implications on localisation and function.Mol Biol Cell. 2011 Jan 1;22(1):91-104. doi: 10.1091/mbc.E10-06-0543. Epub 2010 Nov 30. Mol Biol Cell. 2011. PMID: 21119002 Free PMC article.
-
A complex containing the CPSF73 endonuclease and other polyadenylation factors associates with U7 snRNP and is recruited to histone pre-mRNA for 3'-end processing.Mol Cell Biol. 2013 Jan;33(1):28-37. doi: 10.1128/MCB.00653-12. Epub 2012 Oct 15. Mol Cell Biol. 2013. PMID: 23071092 Free PMC article.
-
U2 snRNP: not just for poly(A) mRNAs.Mol Cell. 2007 Nov 9;28(3):353-4. doi: 10.1016/j.molcel.2007.10.015. Mol Cell. 2007. PMID: 17996698 Review.
-
U7 deciphered: the mechanism that forms the unusual 3' end of metazoan replication-dependent histone mRNAs.Biochem Soc Trans. 2021 Nov 1;49(5):2229-2240. doi: 10.1042/BST20210323. Biochem Soc Trans. 2021. PMID: 34351387 Free PMC article. Review.
Cited by
-
Generation of plasmid vectors expressing FLAG-tagged proteins under the regulation of human elongation factor-1α promoter using Gibson assembly.J Vis Exp. 2015 Feb 9;(96):52235. doi: 10.3791/52235. J Vis Exp. 2015. PMID: 25742071 Free PMC article.
-
TauCstF-64 Mediates Correct mRNA Polyadenylation and Splicing of Activator and Repressor Isoforms of the Cyclic AMP-Responsive Element Modulator (CREM) in Mouse Testis.Biol Reprod. 2016 Feb;94(2):34. doi: 10.1095/biolreprod.115.134684. Epub 2015 Dec 23. Biol Reprod. 2016. PMID: 26700942 Free PMC article.
-
Multi-faceted quantitative proteomics analysis of histone H2B isoforms and their modifications.Epigenetics Chromatin. 2015 Apr 22;8:15. doi: 10.1186/s13072-015-0006-8. eCollection 2015. Epigenetics Chromatin. 2015. PMID: 25922622 Free PMC article.
-
The structural basis of CstF-77 modulation of cleavage and polyadenylation through stimulation of CstF-64 activity.Nucleic Acids Res. 2018 Dec 14;46(22):12022-12039. doi: 10.1093/nar/gky862. Nucleic Acids Res. 2018. PMID: 30257008 Free PMC article.
-
CstF-64 is necessary for endoderm differentiation resulting in cardiomyocyte defects.Stem Cell Res. 2014 Nov;13(3 Pt A):413-21. doi: 10.1016/j.scr.2014.09.005. Epub 2014 Sep 28. Stem Cell Res. 2014. PMID: 25460602 Free PMC article.
References
-
- Coronado D., Godet M., Bourillot P.Y., Tapponnier Y., Bernat A., Petit M., Afanassieff M., Markossian S., Malashicheva A., Iacone R., et al. A short G1 phase is an intrinsic determinant of naive embryonic stem cell pluripotency. Stem Cell Res. 2013;10:118–131. - PubMed
-
- Ghule P.N., Medina R., Lengner C.J., Mandeville M., Qiao M., Dominski Z., Lian J.B., Stein J.L., van Wijnen A.J., Stein G.S. Reprogramming the pluripotent cell cycle: restoration of an abbreviated G1 phase in human induced pluripotent stem (iPS) cells. J. Cell. Physiol. 2011;226:1149–1156. - PMC - PubMed
-
- Becker K.A., Stein J.L., Lian J.B., van Wijnen A.J., Stein G.S. Establishment of histone gene regulation and cell cycle checkpoint control in human embryonic stem cells. J. Cell. Physiol. 2007;210:517–526. - PubMed
-
- Koledova Z., Kafkova L.R., Calabkova L., Krystof V., Dolezel P., Divoky V. Cdk2 inhibition prolongs G1 phase progression in mouse embryonic stem cells. Stem Cells Dev. 2010;19:181–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

