Comparative composition and antioxidant activity of Peptide fractions obtained by ultrafiltration of egg yolk protein enzymatic hydrolysates
- PMID: 24957729
- PMCID: PMC4021898
- DOI: 10.3390/membranes1030149
Comparative composition and antioxidant activity of Peptide fractions obtained by ultrafiltration of egg yolk protein enzymatic hydrolysates
Abstract
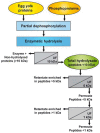
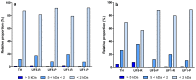
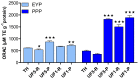
The objective of the study was to compare the antioxidant activity of two distinct hydrolysates and their peptide fractions prepared by ultrafiltration (UF) using membranes with molecular weight cut-off of 5 and 1 kDa. The hydrolysates were a delipidated egg yolk protein concentrate (EYP) intensively hydrolyzed with a combination of two bacterial proteases, and a phosphoproteins (PPP) extract partially hydrolyzed with trypsin. Antioxidant activity, as determined by the oxygen radical absorbance capacity (ORAC) assay, was low for EYP and PPP hydrolysates with values of 613.1 and 489.2 µM TE×g-1 protein, respectively. UF-fractionation of EYP hydrolysate increased slightly the antioxidant activity in permeate fractions (720.5-867.8 µM TE×g-1 protein). However, ORAC values were increased by more than 3-fold in UF-fractions prepared from PPP hydrolysate, which were enriched in peptides with molecular weight lower than 5 kDa. These UF-fractions were characterized by their lower N/P atomic ratio and higher phosphorus content compared to the same UF-fractions obtained from EYP-TH. They also contained high amounts of His, Met, Leu, and Phe, which are recognized as antioxidant amino acids, but also high content in Lys and Arg which both represent target amino acids of trypsin used for the hydrolysis of PPP.
Figures

Similar articles
-
Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white.Food Chem. 2015 Dec 1;188:467-72. doi: 10.1016/j.foodchem.2015.05.014. Epub 2015 May 6. Food Chem. 2015. PMID: 26041219
-
Antioxidant Activity of Egg Yolk Protein Hydrolysates Obtained by Enzymatic and Sub-Critical Water Hydrolysis.Molecules. 2023 Nov 29;28(23):7836. doi: 10.3390/molecules28237836. Molecules. 2023. PMID: 38067564 Free PMC article.
-
Fractionation and identification of novel antioxidant peptides from buffalo and bovine casein hydrolysates.Food Chem. 2017 Oct 1;232:753-762. doi: 10.1016/j.foodchem.2017.04.071. Epub 2017 Apr 13. Food Chem. 2017. PMID: 28490137
-
Antioxidant activities of bambara groundnut (Vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions.Food Funct. 2016 May 18;7(5):2431-7. doi: 10.1039/c6fo00057f. Epub 2016 May 9. Food Funct. 2016. PMID: 27156453
-
Enzymatic Protein Hydrolysates, and Ultrafiltered Peptide Fractions from Two Molluscs: Tympanotonus fuscatus var. radula (L.) and Pachymelania aurita (M.), with Angiotensin-I-Converting Enzyme Inhibitory and DPPH Radical Scavenging Activities.Int J Appl Basic Med Res. 2021 Apr-Jun;11(2):70-74. doi: 10.4103/ijabmr.IJABMR_375_19. Epub 2021 Apr 8. Int J Appl Basic Med Res. 2021. PMID: 33912424 Free PMC article.
Cited by
-
The Impact of Processing and Extraction Methods on the Allergenicity of Targeted Protein Quantification as Well as Bioactive Peptides Derived from Egg.Molecules. 2023 Mar 15;28(6):2658. doi: 10.3390/molecules28062658. Molecules. 2023. PMID: 36985630 Free PMC article. Review.
-
Egg Yolk as a New Source of Peptides with Antioxidant and Antimicrobial Properties.Foods. 2023 Sep 11;12(18):3394. doi: 10.3390/foods12183394. Foods. 2023. PMID: 37761103 Free PMC article.
-
Multifunctional peptides derived from an egg yolk protein hydrolysate: isolation and characterization.Amino Acids. 2015 Feb;47(2):369-80. doi: 10.1007/s00726-014-1869-x. Epub 2014 Nov 20. Amino Acids. 2015. PMID: 25408464 Free PMC article.
-
Purification and Characterization of an Antioxidant Protein from Fertilized Eggs.Korean J Food Sci Anim Resour. 2016;36(6):791-798. doi: 10.5851/kosfa.2016.36.6.791. Epub 2016 Dec 31. Korean J Food Sci Anim Resour. 2016. PMID: 28115891 Free PMC article.
-
Purification and Identification of a Natural Antioxidant Protein from Fertilized Eggs.Korean J Food Sci Anim Resour. 2017;37(5):764-772. doi: 10.5851/kosfa.2017.37.5.764. Epub 2017 Oct 31. Korean J Food Sci Anim Resour. 2017. PMID: 29147100 Free PMC article.
References
-
- Decker E.A., Warner K., Richards M.P., Shahidi F. Measuring antioxidant effectiveness in food. J. Agric. Food Chem. 2005;53:4303–4310. - PubMed
-
- Ito N., Hirose M., Fukushima S., Tsuda H., Shirai T., Tatematsu M. Studies on antioxidant: Their carcinogenic and modifying effects on chemical carcinogenic. Food Chem. Toxicol. 1986;24:1071–1082. - PubMed
-
- Seifried H.E., Anderson D.E., Fisher E.I., Milner J.A. A review of the interaction among antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007;18:567–579. - PubMed
-
- Sarmadi B.H., Ismail A. Antioxidative peptides from food proteins: A review. Peptides. 2010;31:1949–1956. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

