Changes in primary and secondary metabolite levels in response to gene targeting-mediated site-directed mutagenesis of the anthranilate synthase gene in rice
- PMID: 24957777
- PMCID: PMC3901229
- DOI: 10.3390/metabo2041123
Changes in primary and secondary metabolite levels in response to gene targeting-mediated site-directed mutagenesis of the anthranilate synthase gene in rice
Abstract
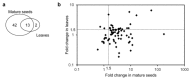
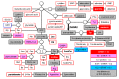
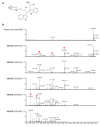
Gene targeting (GT) via homologous recombination allows precise modification of a target gene of interest. In a previous study, we successfully used GT to produce rice plants accumulating high levels of free tryptophan (Trp) in mature seeds and young leaves via targeted modification of a gene encoding anthranilate synthase-a key enzyme of Trp biosynthesis. Here, we performed metabolome analysis in the leaves and mature seeds of GT plants. Of 72 metabolites detected in both organs, a total of 13, including Trp, involved in amino acid metabolism, accumulated to levels >1.5-fold higher than controls in both leaves and mature seeds of GT plants. Surprisingly, the contents of certain metabolites valuable for both humans and livestock, such as γ-aminobutyric acid and vitamin B, were significantly increased in mature seeds of GT plants. Moreover, untargeted analysis using LC-MS revealed that secondary metabolites, including an indole alkaloid, 2-[2-hydroxy-3-β-d-glucopyranosyloxy-1-(1H-indol-3-yl)propyl] tryptophan, also accumulate to higher levels in GT plants. Some of these metabolite changes in plants produced via GT are similar to those observed in plants over expressing mutated genes, thus demonstrating that in vivo protein engineering via GT can be an effective approach to metabolic engineering in crops.
Figures
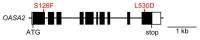


References
-
- Ye X.D., Al-Babili S., Kloti A., Zhang J., Lucca P., Beyer P., Potrykus I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287:303–305. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

