Modulation of insulin dose titration using a hypoglycaemia-sensitive algorithm: insulin glargine versus neutral protamine Hagedorn insulin in insulin-naïve people with type 2 diabetes
- PMID: 24957785
- PMCID: PMC4282751
- DOI: 10.1111/dom.12329
Modulation of insulin dose titration using a hypoglycaemia-sensitive algorithm: insulin glargine versus neutral protamine Hagedorn insulin in insulin-naïve people with type 2 diabetes
Abstract
Aims: To examine whether insulin glargine can lead to better control of glycated haemoglobin (HbA1c) than that achieved by neutral protamine Hagedorn (NPH) insulin, using a protocol designed to limit nocturnal hypoglycaemia.
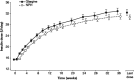
Methods: The present study, the Least One Oral Antidiabetic Drug Treatment (LANCELOT) Study, was a 36-week, randomized, open-label, parallel-arm study conducted in Europe, Asia, the Middle East and South America. Participants were randomized (1:1) to begin glargine or NPH, on background of metformin with glimepiride. Weekly insulin titration aimed to achieve median prebreakfast and nocturnal plasma glucose levels ≤5.5 mmol/l, while limiting values ≤4.4 mmol/l.
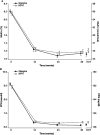
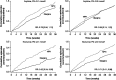
Results: The efficacy population (n = 701) had a mean age of 57 years, a mean body mass index of 29.8 kg/m², a mean duration of diabetes of 9.2 years and a mean HbA1c level of 8.2% (66 mmol/mol). At treatment end, HbA1c values and the proportion of participants with HbA1c <7.0 % (<53 mmol/mol) were not significantly different for glargine [7.1 % (54 mmol/mol) and 50.3%] versus NPH [7.2 % (55 mmol/mol) and 44.3%]. The rate of symptomatic nocturnal hypoglycaemia, confirmed by plasma glucose ≤3.9 or ≤3.1 mmol/l, was 29 and 48% less with glargine than with NPH insulin. Other outcomes were similar between the groups.
Conclusion: Insulin glargine was not superior to NPH insulin in improving glycaemic control. The insulin dosing algorithm was not sufficient to equalize nocturnal hypoglycaemia between the two insulins. This study confirms, in a globally heterogeneous population, the reduction achieved in nocturnal hypoglycaemia while attaining good glycaemic control with insulin glargine compared with NPH, even when titrating basal insulin to prevent nocturnal hypoglycaemia rather than treating according to normal fasting glucose levels.
Trial registration: ClinicalTrials.gov NCT00949442.
Keywords: NPH insulin; hypoglycaemia-sensitive algorithm; insulin glargine.
© 2014 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
References
-
- International Diabetes Federation. IDF Clinical Guidelines Task Force. 2012. Global guideline for type 2 diabetes. International Diabetes Federation Web site. Available from URL: https://www.aace.com/files/dm-guidelines-ccp.pdf. Accessed 3 April 2013.
-
- NICE. 2013. Blood-glucose-lowering therapy for type 2 diabetes. Available from URL: http://pathways.nice.org.uk/pathways/diabetes/blood-glucose-lowering-the.... Accessed 6 December.
-
- Rodbard HW, Blonde L, Braithwaite SS. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13(Suppl. 1):1–68. et al. - PubMed
-
- Hermansen K, Davies M, Derezinski T, Martinez RG, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–1274. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

