Phase I safety, pharmacokinetics, and pharmacogenetics study of the antituberculosis drug PA-824 with concomitant lopinavir-ritonavir, efavirenz, or rifampin
- PMID: 24957823
- PMCID: PMC4135849
- DOI: 10.1128/AAC.03332-14
Phase I safety, pharmacokinetics, and pharmacogenetics study of the antituberculosis drug PA-824 with concomitant lopinavir-ritonavir, efavirenz, or rifampin
Abstract
There is an urgent need for new antituberculosis (anti-TB) drugs, including agents that are safe and effective with concomitant antiretrovirals (ARV) and first-line TB drugs. PA-824 is a novel antituberculosis nitroimidazole in late-phase clinical development. Cytochrome P450 (CYP) 3A, which can be induced or inhibited by ARV and antituberculosis drugs, is a minor (∼20%) metabolic pathway for PA-824. In a phase I clinical trial, we characterized interactions between PA-824 and efavirenz (arm 1), lopinavir/ritonavir (arm 2), and rifampin (arm 3) in healthy, HIV-uninfected volunteers without TB disease. Participants in arms 1 and 2 were randomized to receive drugs via sequence 1 (PA-824 alone, washout, ARV, and ARV plus PA-824) or sequence 2 (ARV, ARV with PA-824, washout, and PA-824 alone). In arm 3, participants received PA-824 and then rifampin and then both. Pharmacokinetic sampling occurred at the end of each dosing period. Fifty-two individuals participated. Compared to PA-824 alone, plasma PA-824 values (based on geometric mean ratios) for maximum concentration (Cmax), area under the concentration-time curve from 0 to 24 h (AUC0-24), and trough concentration (Cmin) were reduced 28%, 35%, and 46% with efavirenz, 13%, 17%, and 21% with lopinavir-ritonavir (lopinavir/r) and 53%, 66%, and 85% with rifampin, respectively. Medications were well tolerated. In conclusion, lopinavir/r had minimal effect on PA-824 exposures, supporting PA-824 use with lopinavir/r without dose adjustment. PA-824 exposures, though, were reduced more than expected when given with efavirenz or rifampin. The clinical implications of these reductions will depend upon data from current clinical trials defining PA-824 concentration-effect relationships. (This study has been registered at ClinicalTrials.gov under registration no. NCT01571414.).
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
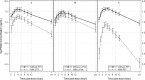
References
-
- World Health Organization. 2013. Global tuberculosis report 2013. WHO/HTM/TB/2013.11. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/
-
- World Health Organization. 2013. Multidrug-resistant tuberculosis (MDR-TB), 2013 update. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/challenges/mdr/MDR_TB_FactSheet.pdf
-
- World Health Organization. 2011. Guidelines for the programmatic management of drug-resistant tuberculosis, 2011 update. WHO/HTM/TB/2011.6. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/challenges/mdr/programmatic_guidelines_for_mdrtb/e... - PubMed
-
- Lenaerts AJ, Gruppo V, Marietta KS, Johnson CM, Driscoll DK, Tompkins NM, Rose JD, Reynolds RC, Orme IM. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294–2301. 10.1128/AAC.49.6.2294-2301.2005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01-AI069502-01/AI/NIAID NIH HHS/United States
- R01 AI077505/AI/NIAID NIH HHS/United States
- U01-AI069439-01/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- 1U01AI069474-01/AI/NIAID NIH HHS/United States
- HHSN272200800014C/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 AI069502/AI/NIAID NIH HHS/United States
- UL1 TR001070/TR/NCATS NIH HHS/United States
- UM1AI68634/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- TR-000445/TR/NCATS NIH HHS/United States
- U01 AI069474/AI/NIAID NIH HHS/United States
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- U01 AI069465/AI/NIAID NIH HHS/United States
- UL1TR001070/TR/NCATS NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- K23 AI080842/AI/NIAID NIH HHS/United States
- AI-077505/AI/NIAID NIH HHS/United States
- 1U01AI069465-01/AI/NIAID NIH HHS/United States
- UL1RR024975/RR/NCRR NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- UL1TR000004/TR/NCATS NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- K23AI080842/AI/NIAID NIH HHS/United States
- UM1AI068636/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

