Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond
- PMID: 24957944
- PMCID: PMC4250230
- DOI: 10.1038/nrc3760
Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond
Abstract
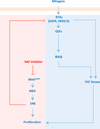
The identification of mutationally activated BRAF in many cancers altered our conception of the part played by the RAF family of protein kinases in oncogenesis. In this Review, we describe the development of BRAF inhibitors and the results that have emerged from their analysis in both the laboratory and the clinic. We discuss the spectrum of RAF mutations in human cancer and the complex interplay between the tissue of origin and the response to RAF inhibition. Finally, we enumerate mechanisms of resistance to BRAF inhibition that have been characterized and postulate how strategies of RAF pathway inhibition may be extended in scope to benefit not only the thousands of patients who are diagnosed annually with BRAF-mutated metastatic melanoma but also the larger patient population with malignancies harbouring mutationally activated RAF genes that are ineffectively treated with the current generation of BRAF kinase inhibitors.
Figures

References
-
- Moelling K, Heimann B, Beimling P, Rapp UR, Sander T. Serine- and threonine-specific protein kinase activities of purified gag-mil and gag-raf proteins. Nature. 1984;312:558–561. - PubMed
-
- Bonner T, et al. The human homologs of the raf (mil) oncogene are located on human chromosomes 3 and 4. Science. 1984;223:71–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

