The BAR Domain Superfamily Proteins from Subcellular Structures to Human Diseases
- PMID: 24957964
- PMCID: PMC4021885
- DOI: 10.3390/membranes2010091
The BAR Domain Superfamily Proteins from Subcellular Structures to Human Diseases
Abstract
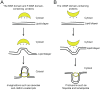
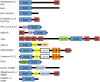
Eukaryotic cells have complicated membrane systems. The outermost plasma membrane contains various substructures, such as invaginations and protrusions, which are involved in endocytosis and cell migration. Moreover, the intracellular membrane compartments, such as autophagosomes and endosomes, are essential for cellular viability. The Bin-Amphiphysin-Rvs167 (BAR) domain superfamily proteins are important players in membrane remodeling through their structurally determined membrane binding surfaces. A variety of BAR domain superfamily proteins exist, and each family member appears to be involved in the formation of certain subcellular structures or intracellular membrane compartments. Most of the BAR domain superfamily proteins contain SH3 domains, which bind to the membrane scission molecule, dynamin, as well as the actin regulatory WASP/WAVE proteins and several signal transduction molecules, providing possible links between the membrane and the cytoskeleton or other machineries. In this review, we summarize the current information about each BAR superfamily protein with an SH3 domain(s). The involvement of BAR domain superfamily proteins in various diseases is also discussed.
Figures



References
-
- Suetsugu S., Toyooka K., Senju Y. Subcellular membrane curvature mediated by the BAR domain superfamily proteins. Semin. Cell Dev. Biol. 2010;21:340–349. - PubMed
LinkOut - more resources
Full Text Sources

