Retrospective study of carmustine or lomustine with bevacizumab in recurrent glioblastoma patients who have failed prior bevacizumab
- PMID: 24958095
- PMCID: PMC4201074
- DOI: 10.1093/neuonc/nou118
Retrospective study of carmustine or lomustine with bevacizumab in recurrent glioblastoma patients who have failed prior bevacizumab
Abstract
Background: Currently, there are no known effective treatments for recurrent glioblastoma once patients have progressed on a bevacizumab-containing regimen. We examined the efficacy of adding nitrosoureas to bevacizumab in patients who progressed while on an initial bevacizumab-containing regimen.
Methods: In this retrospective study, we identified adult patients with histologically confirmed glioblastoma (WHO grade IV) who were treated with lomustine or carmustine in combination with bevacizumab as a second or third regimen after failing an alternative initial bevacizumab-containing regimen. Response rate (RR), 6-month progression free survival (PFS6), and progression-free survival (PFS) were assessed for each treatment.
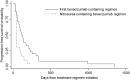
Results: Forty-two patients were identified (28 males) with a median age of 49 years (range, 24-78 y). Of 42 patients, 28 received lomustine (n = 22) or carmustine (n = 6) with bevacizumab as their second bevacizumab-containing regimen, and 14 received lomustine (n = 11) or carmustine (n = 3) as their third bevacizumab-containing regimen. While the median PFS for the initial bevacizumab-containing regimen was 16.3 weeks, the median PFS for the nitrosourea-containing bevacizumab regimen was 6.3 weeks. Patients had an RR of 44% and a PFS6 rate of 26% during the initial bevacizumab regimen and an RR of 0% and a PFS6 rate of 3% during the nitrosourea-containing bevacizumab regimen. There was increased grade 3-4 toxicity (45% vs 19%, P = .010) during the nitrosourea-containing bevacizumab regimen relative to the initial bevacizumab regimen. Median overall survival was 18.7 weeks from initiation of the nitrosourea-containing bevacizumab regimen.
Conclusion: The addition of lomustine or carmustine to bevacizumab after a patient has already progressed on a bevacizumab-containing regimen does not appear to provide benefit for most patients and is associated with additional toxicity with the doses used in this cohort.
Keywords: bevacizumab; malignant glioma; nitrosoureas; recurrent glioblastoma.
© The Author(s) 2014. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Timing (and biology) are everything.Neuro Oncol. 2014 Nov;16(11):1431-2. doi: 10.1093/neuonc/nou285. Epub 2014 Oct 14. Neuro Oncol. 2014. PMID: 25314963 Free PMC article. No abstract available.
References
-
- 2000. Central Brain Tumor Registry of the United States: Statistical report: Primary brain tumors in the United States. Available at: http://www.cbtrus.org/reports/2007-2008/2007report.pdf .
-
- Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. - PubMed
-
- Norden AD, Drappatz J, Muzikansky A, et al. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol. 2009;92(2):149–155. - PubMed
-
- 2012. Genentech, Inc. Avastin (Highlights of Prescribing Information). Available at http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

