Novel functional complexity of polycystin-1 by GPS cleavage in vivo: role in polycystic kidney disease
- PMID: 24958103
- PMCID: PMC4135549
- DOI: 10.1128/MCB.00687-14
Novel functional complexity of polycystin-1 by GPS cleavage in vivo: role in polycystic kidney disease
Abstract
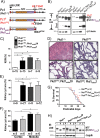
Polycystin-1 (Pc1) cleavage at the G protein-coupled receptor (GPCR) proteolytic site (GPS) is required for normal kidney morphology in humans and mice. We found a complex pattern of endogenous Pc1 forms by GPS cleavage. GPS cleavage generates not only the heterodimeric cleaved full-length Pc1 (Pc1(cFL)) in which the N-terminal fragment (NTF) remains noncovalently associated with the C-terminal fragment (CTF) but also a novel (Pc1) form (Pc1(deN)) in which NTF becomes detached from CTF. Uncleaved Pc1 (Pc1(U)) resides primarily in the endoplasmic reticulum (ER), whereas both Pc1(cFL) and Pc1(deN) traffic through the secretory pathway in vivo. GPS cleavage is not a prerequisite, however, for Pc1 trafficking in vivo. Importantly, Pc1(deN) is predominantly found at the plasma membrane of renal epithelial cells. By functional genetic complementation with five Pkd1 mouse models, we discovered that CTF plays a crucial role in Pc1(deN) trafficking. Our studies support GPS cleavage as a critical regulatory mechanism of Pc1 biogenesis and trafficking for proper kidney development and homeostasis.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
