Investigation of cross-linked and additive containing polymer materials for membranes with improved performance in pervaporation and gas separation
- PMID: 24958427
- PMCID: PMC4021918
- DOI: 10.3390/membranes2040727
Investigation of cross-linked and additive containing polymer materials for membranes with improved performance in pervaporation and gas separation
Abstract
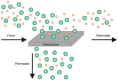
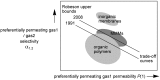
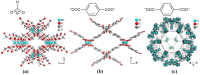
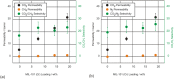
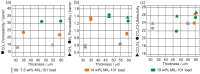
Pervaporation and gas separation performances of polymer membranes can be improved by crosslinking or addition of metal-organic frameworks (MOFs). Crosslinked copolyimide membranes show higher plasticization resistance and no significant loss in selectivity compared to non-crosslinked membranes when exposed to mixtures of CO2/CH4 or toluene/cyclohexane. Covalently crosslinked membranes reveal better separation performances than ionically crosslinked systems. Covalent interlacing with 3-hydroxypropyldimethylmaleimide as photocrosslinker can be investigated in situ in solution as well as in films, using transient UV/Vis and FTIR spectroscopy. The photocrosslinking yield can be determined from the FTIR-spectra. It is restricted by the stiffness of the copolyimide backbone, which inhibits the photoreaction due to spatial separation of the crosslinker side chains. Mixed-matrix membranes (MMMs) with MOFs as additives (fillers) have increased permeabilities and often also selectivities compared to the pure polymer. Incorporation of MOFs into polysulfone and Matrimid® polymers for MMMs gives defect-free membranes with performances similar to the best polymer membranes for gas mixtures, such as O2/N2 H2/CH4, CO2/CH4, H2/CO2, CH4/N2 and CO2/N2 (preferentially permeating gas is named first). The MOF porosity, its particle size and content in the MMM are factors to influence the permeability and the separation performance of the membranes.
Figures








Similar articles
-
Computational Investigation of Dual Filler-Incorporated Polymer Membranes for Efficient CO2 and H2 Separation: MOF/COF/Polymer Mixed Matrix Membranes.Ind Eng Chem Res. 2023 Jan 26;62(6):2924-2936. doi: 10.1021/acs.iecr.2c04500. eCollection 2023 Feb 15. Ind Eng Chem Res. 2023. PMID: 36812129 Free PMC article.
-
Metal-organic frameworks in mixed-matrix membranes for gas separation.Dalton Trans. 2012 Dec 14;41(46):14003-27. doi: 10.1039/c2dt31550e. Epub 2012 Oct 16. Dalton Trans. 2012. PMID: 23070078
-
Molecular Simulations of MOF Membranes and Performance Predictions of MOF/Polymer Mixed Matrix Membranes for CO2/CH4 Separations.ACS Sustain Chem Eng. 2019 Jan 22;7(2):2739-2750. doi: 10.1021/acssuschemeng.8b05832. Epub 2018 Dec 18. ACS Sustain Chem Eng. 2019. PMID: 30701144 Free PMC article.
-
Metal Organic Framework - Based Mixed Matrix Membranes for Carbon Dioxide Separation: Recent Advances and Future Directions.Front Chem. 2020 Jul 3;8:534. doi: 10.3389/fchem.2020.00534. eCollection 2020. Front Chem. 2020. PMID: 32719772 Free PMC article. Review.
-
Metal-Organic-Frameworks Based Mixed-Matrix Membranes for CO2 Separation: An Applicable-Conceptual Approach.ACS Appl Mater Interfaces. 2024 Jul 3;16(26):32906-32929. doi: 10.1021/acsami.4c06914. Epub 2024 Jun 22. ACS Appl Mater Interfaces. 2024. PMID: 38907700 Review.
Cited by
-
Preparation and Characterization of Cellulose Acetate Propionate Films Functionalized with Reactive Ionic Liquids.Polymers (Basel). 2019 Jul 20;11(7):1217. doi: 10.3390/polym11071217. Polymers (Basel). 2019. PMID: 31330836 Free PMC article.
-
Infiltrated thin film structure with hydrogel-mediated precursor ink for durable SOFCs.Sci Rep. 2021 Mar 29;11(1):7109. doi: 10.1038/s41598-021-86572-w. Sci Rep. 2021. PMID: 33782467 Free PMC article.
-
Carbon dioxide separation from flue gases: a technological review emphasizing reduction in greenhouse gas emissions.ScientificWorldJournal. 2014 Feb 17;2014:828131. doi: 10.1155/2014/828131. eCollection 2014. ScientificWorldJournal. 2014. PMID: 24696663 Free PMC article. Review.
-
Membrane-Introduction Mass Spectrometry Analysis of Desflurane, Propofol and Fentanyl in Plasma and Cerebrospinal Fluid for Estimation BBB Properties.Exp Neurobiol. 2015 Sep;24(3):206-10. doi: 10.5607/en.2015.24.3.206. Epub 2015 Sep 22. Exp Neurobiol. 2015. PMID: 26412969 Free PMC article.
-
Carbon Dioxide Capture and Conversion Using Metal-Organic Framework (MOF) Materials: A Comprehensive Review.Nanomaterials (Basel). 2024 Aug 12;14(16):1340. doi: 10.3390/nano14161340. Nanomaterials (Basel). 2024. PMID: 39195378 Free PMC article. Review.
References
-
- King C.J. Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley and Sons, Inc.; Hoboken, NJ, USA: 2000. Separation Processes, Introduction.
-
- Davis J.C., Valus R.J., Eshraghi R., Velikoff A.E. Facilitated transport membrane hybrid systems for olefin purification. Sep. Sci. Tec. 1993;28:463–476. doi: 10.1080/01496399308019500. - DOI
-
- Kondo M., Komori M., Kita H., Okamoto K. Tubular-type pervaporation module with zeolite NaA membrane. J. Membrane Sci. 1997;133:133–141. doi: 10.1016/S0376-7388(97)00087-2. - DOI
-
- Holmes S.M., Schmitt M., Markert C., Plaisted R.J., Forrest J.O., Sharratt P.N., Garforth A.A., Cundy C.S., Dwyer J. Zeolite A membranes for use in alcohol/water separations—Part I: Experimental investigation. Chem. Eng. Res. Des. 2000;78:1084–1088. doi: 10.1205/026387600528355. - DOI
-
- Van den Berg A.W.C., Gora L., Jansen J.C., Makkee M., Maschmeyer T. Zeolite A membranes synthesized on a UV-irradiated TiO2 coated metal support: The high pervaporation performance. J. Membrane Sci. 2003;224:29–37. doi: 10.1016/S0376-7388(03)00345-4. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

