Chronic intermittent hypoxia and hypercapnia inhibit the hypothalamic paraventricular nucleus neurotransmission to parasympathetic cardiac neurons in the brain stem
- PMID: 24958501
- PMCID: PMC4134391
- DOI: 10.1161/HYPERTENSIONAHA.114.03603
Chronic intermittent hypoxia and hypercapnia inhibit the hypothalamic paraventricular nucleus neurotransmission to parasympathetic cardiac neurons in the brain stem
Abstract
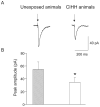
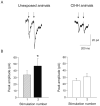
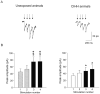
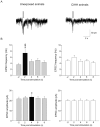
Obstructive sleep apnea is associated with chronic intermittent hypoxia/hypercapnia (CIHH) episodes during sleep that heighten sympathetic and diminish parasympathetic activity to the heart. Although one population of neurons in the paraventricular nucleus of the hypothalamus strongly influences sympathetic tone and has increased activity after CIHH, little is known about the role of this pathway to parasympathetic neurons and how this network is altered in CIHH. We hypothesized that CIHH inhibits the excitatory pathway from the paraventricular nucleus of the hypothalamus to parasympathetic cardiac vagal neurons in the brain stem. To test this hypothesis, channelrhodopsin was selectively expressed, using viral vectors, in neurons in the paraventricular nucleus of the hypothalamus and channelrhodopsin-expressing fibers were photoactivated to evoke postsynaptic currents in cardiac vagal neurons in brain stem slices. Excitatory postsynaptic currents were diminished in animals exposed to CIHH. The paired-pulse and prolonged facilitation of the postsynaptic current amplitudes and frequencies evoked by paired and bursts of photoactivation of channelrhodopsin fibers, respectively, occurred in unexposed rats but were blunted in CIHH animals. In response to an acute challenge of hypoxia/hypercapnia, the amplitude of postsynaptic events was unchanged during, but increased after hypoxia/hypercapnia in unexposed animals. In contrast, postsynaptic currents were inhibited during hypoxia/hypercapnia in rats exposed to CIHH. In conclusion, the excitatory pathway to cardiac vagal neurons is diminished in response to both acute and chronic exposures to hypoxia/hypercapnia. This could elicit a reduced cardioprotective parasympathetic activity and an enhanced risk of adverse cardiovascular events in episodes of apnea and chronic obstructive sleep apnea.
Keywords: anoxia; hypercapnia; hypothalamus.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Figures
Similar articles
-
Chronic intermittent hypoxia alters neurotransmission from lateral paragigantocellular nucleus to parasympathetic cardiac neurons in the brain stem.J Neurophysiol. 2015 Jan 1;113(1):380-9. doi: 10.1152/jn.00302.2014. Epub 2014 Oct 15. J Neurophysiol. 2015. PMID: 25318765
-
Chronic intermittent hypoxia-hypercapnia blunts heart rate responses and alters neurotransmission to cardiac vagal neurons.J Physiol. 2014 Jul 1;592(13):2799-811. doi: 10.1113/jphysiol.2014.273482. Epub 2014 May 16. J Physiol. 2014. PMID: 24835174 Free PMC article.
-
Combined hypoxia and hypercapnia, but not hypoxia alone, suppresses neurotransmission from orexin to hypothalamic paraventricular spinally-projecting neurons in weanling rats.Brain Res. 2018 Jan 15;1679:33-38. doi: 10.1016/j.brainres.2017.11.015. Epub 2017 Nov 21. Brain Res. 2018. PMID: 29162453 Free PMC article.
-
Function and modulation of premotor brainstem parasympathetic cardiac neurons that control heart rate by hypoxia-, sleep-, and sleep-related diseases including obstructive sleep apnea.Prog Brain Res. 2014;212:39-58. doi: 10.1016/B978-0-444-63488-7.00003-3. Prog Brain Res. 2014. PMID: 25194192 Review.
-
The sympathetic nervous system and obstructive sleep apnea: implications for hypertension.J Hypertens. 1997 Dec;15(12 Pt 2):1613-9. doi: 10.1097/00004872-199715120-00062. J Hypertens. 1997. PMID: 9488212 Review.
Cited by
-
Breathing disturbances in Rett syndrome.Handb Clin Neurol. 2022;189:139-151. doi: 10.1016/B978-0-323-91532-8.00018-5. Handb Clin Neurol. 2022. PMID: 36031301 Free PMC article. Review.
-
Experimental Models to Study End-Organ Morbidity in Sleep Apnea: Lessons Learned and Future Directions.Int J Mol Sci. 2022 Nov 20;23(22):14430. doi: 10.3390/ijms232214430. Int J Mol Sci. 2022. PMID: 36430904 Free PMC article. Review.
-
Molecular and cellular neurocardiology in heart disease.J Physiol. 2025 Mar;603(7):1689-1728. doi: 10.1113/JP284739. Epub 2024 May 22. J Physiol. 2025. PMID: 38778747 Review.
-
Sleep Apnea Research in Animals. Past, Present, and Future.Am J Respir Cell Mol Biol. 2016 Mar;54(3):299-305. doi: 10.1165/rcmb.2015-0218TR. Am J Respir Cell Mol Biol. 2016. PMID: 26448201 Free PMC article. Review.
-
Oxytocin neuron activation prevents hypertension that occurs with chronic intermittent hypoxia/hypercapnia in rats.Am J Physiol Heart Circ Physiol. 2016 Jun 1;310(11):H1549-57. doi: 10.1152/ajpheart.00808.2015. Epub 2016 Mar 25. Am J Physiol Heart Circ Physiol. 2016. PMID: 27016581 Free PMC article.
References
-
- Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–423. - PubMed
-
- Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79:1036–1046. - PubMed
-
- Kato M, Adachi T, Koshino Y, Somers VK. Obstructive sleep apnea and cardiovascular disease. Circ J. 2009;73:1363–1370. - PubMed
-
- Parati G, Di Rienzo M, Bonsignore MR, Insalaco G, Marrone O, Castiglioni P, Bonsignore G, Mancia G. Autonomic cardiac regulation in obstructive sleep apnea syndrome: Evidence from spontaneous baroreflex analysis during sleep. J Hypertens. 1997;15:1621–1626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

