Multiple length peptide-pheromone variants produced by Streptococcus pyogenes directly bind Rgg proteins to confer transcriptional regulation
- PMID: 24958729
- PMCID: PMC4139249
- DOI: 10.1074/jbc.M114.583989
Multiple length peptide-pheromone variants produced by Streptococcus pyogenes directly bind Rgg proteins to confer transcriptional regulation
Abstract
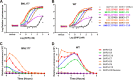
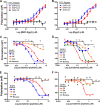
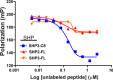
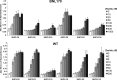
Streptococcus pyogenes, a human-restricted pathogen, accounts for substantial mortality related to infections worldwide. Recent studies indicate that streptococci produce and respond to several secreted peptide signaling molecules (pheromones), including those known as short hydrophobic peptides (SHPs), to regulate gene expression by a quorum-sensing mechanism. Upon transport into the bacterial cell, pheromones bind to and modulate activity of receptor proteins belonging to the Rgg family of transcription factors. Previously, we reported biofilm regulation by the Rgg2/3 quorum-sensing circuit in S. pyogenes. The aim of this study was to identify the composition of mature pheromones from cell-free culture supernatants that facilitate biofilm formation. Bioluminescent reporters were employed to detect active pheromones in culture supernatants fractionated by reverse-phase chromatography, and mass spectrometry was used to characterize their properties. Surprisingly, multiple SHPs that varied by length were detected. Synthetic peptides of each variant were tested individually using bioluminescence reporters and biofilm growth assays, and although activities differed widely among the group, peptides comprising the C-terminal eight amino acids of the full-length native peptide were most active. Direct Rgg/SHP interactions were determined using a fluorescence polarization assay that utilized FITC-labeled peptide ligands. Peptide receptor affinities were seen to be as low as 500 nm and their binding affinities directly correlated with observed bioactivity. Revelation of naturally produced pheromones along with determination of their affinity for cognate receptors are important steps forward in designing compounds whose purpose is positioned for future therapeutics aimed at treating infections through the interference of bacterial communication.
Keywords: Biofilm; Fluorescence Anisotropy; Gram-positive Bacteria; Pheromone; Quorum Sensing; Rgg; SHP; Streptococcus pyogenes (S. pyogenes).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Waters C. M., Bassler B. L. (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346 - PubMed
-
- Antunes L. C., Ferreira R. B., Buckner M. M., Finlay B. B. (2010) Quorum sensing in bacterial virulence. Microbiology 156, 2271–2282 - PubMed
-
- Romero M., Acuña L., Otero A. (2012) Patents on quorum quenching: interfering with bacterial communication as a strategy to fight infections. Recent Pat. Biotechnol. 6, 2–12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

