SLK-dependent activation of ERMs controls LGN-NuMA localization and spindle orientation
- PMID: 24958772
- PMCID: PMC4068135
- DOI: 10.1083/jcb.201401049
SLK-dependent activation of ERMs controls LGN-NuMA localization and spindle orientation
Abstract
Mitotic spindle orientation relies on a complex dialog between the spindle microtubules and the cell cortex, in which F-actin has been recently implicated. Here, we report that the membrane-actin linkers ezrin/radixin/moesin (ERMs) are strongly and directly activated by the Ste20-like kinase at mitotic entry in mammalian cells. Using microfabricated adhesive substrates to control the axis of cell division, we found that the activation of ERMs plays a key role in guiding the orientation of the mitotic spindle. Accordingly, impairing ERM activation in apical progenitors of the mouse embryonic neocortex severely disturbed spindle orientation in vivo. At the molecular level, ERM activation promotes the polarized association at the mitotic cortex of leucine-glycine-asparagine repeat protein (LGN) and nuclear mitotic apparatus (NuMA) protein, two essential factors for spindle orientation. We propose that activated ERMs, together with Gαi, are critical for the correct localization of LGN-NuMA force generator complexes and hence for proper spindle orientation.
© 2014 Machicoane et al.
Figures
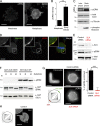
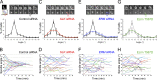
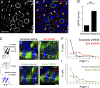
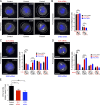

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

