STAT1-induced ASPP2 transcription identifies a link between neuroinflammation, cell polarity, and tumor suppression
- PMID: 24958857
- PMCID: PMC4103354
- DOI: 10.1073/pnas.1407898111
STAT1-induced ASPP2 transcription identifies a link between neuroinflammation, cell polarity, and tumor suppression
Abstract
Inflammation and loss of cell polarity play pivotal roles in neurodegeneration and cancer. A central question in both diseases is how the loss of cell polarity is sensed by cell death machinery. Here, we identify apoptosis-stimulating protein of p53 with signature sequences of ankyrin repeat-, SH3 domain-, and proline-rich region-containing protein 2 (ASPP2), a haploinsufficient tumor suppressor, activator of p53, and regulator of cell polarity, as a transcriptional target of signal transducer and activator of transcription 1 (STAT1). LPS induces ASPP2 expression in murine macrophage and microglial cell lines, a human monocyte cell line, and primary human astrocytes in vitro. LPS and IFNs induce ASPP2 transcription through an NF-κB RELA/p65-independent but STAT1-dependent pathway. In an LPS-induced maternal inflammation mouse model, LPS induces nuclear ASPP2 in vivo at the blood-cerebral spinal fluid barrier (the brain's barrier to inflammation), and ASPP2 mediates LPS-induced apoptosis. Consistent with the role of ASPP2 as a gatekeeper to inflammation, ASPP2-deficient brains possess enhanced neuroinflammation. Elevated ASPP2 expression is also observed in mouse models and human neuroinflammatory disease tissue, where ASPP2 was detected in GFAP-expressing reactive astrocytes that coexpress STAT1. Because the ability of ASPP2 to maintain cellular polarity is vital to CNS development, our findings suggest that the identified STAT1/ASPP2 pathway may connect tumor suppression and cell polarity to neuroinflammation.
Keywords: TLR4; TP53BP2; multiple sclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
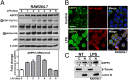
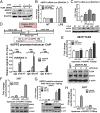

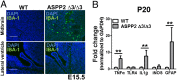

References
-
- Roe CM, Behrens MI, Xiong C, Miller JP, Morris JC. Alzheimer disease and cancer. Neurology. 2005;64(5):895–898. - PubMed
-
- Doody RS, et al. A phase 3 trial of Semagacestat for treatment of Alzheimer's disease. N Engl J Med. 2013;369(4):341–350. - PubMed
-
- Beal MF. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996;6(5):661–666. - PubMed
-
- Davenport CM, Sevastou IG, Hooper C, Pocock JM. Inhibiting p53 pathways in microglia attenuates microglial-evoked neurotoxicity following exposure to Alzheimer peptides. J Neurochem. 2010;112(2):552–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

