Regulation of osteoclast homeostasis and inflammatory bone loss by MFG-E8
- PMID: 24958900
- PMCID: PMC4108495
- DOI: 10.4049/jimmunol.1400970
Regulation of osteoclast homeostasis and inflammatory bone loss by MFG-E8
Abstract
The glycoprotein milk fat globule-epidermal growth factor factor 8 (MFG-E8) is expressed in several tissues and mediates diverse homeostatic functions. However, whether it plays a role in bone homeostasis has not been established. In this study, we show for the first time, to our knowledge, that osteoclasts express and are regulated by MFG-E8. Bone marrow-derived osteoclast precursors from MFG-E8-deficient (Mfge8(-/-)) mice underwent increased receptor activator of NF-κB ligand-induced osteoclastogenesis, leading to enhanced resorption pit formation compared with wild-type controls. Consistently, exogenously added MFG-E8 inhibited receptor activator of NF-κB ligand-induced osteoclastogenesis from mouse or human osteoclast precursors. Upon induction of experimental periodontitis, an oral inflammatory disease characterized by loss of bone support of the dentition, Mfge8(-/-) mice exhibited higher numbers of osteoclasts and more bone loss than did wild-type controls. Accordingly, local microinjection of anti-MFG-E8 mAb exacerbated periodontal bone loss in wild-type mice. Conversely, microinjection of MFG-E8 inhibited bone loss in experimental mouse periodontitis. In comparison with wild-type controls, Mfge8(-/-) mice also experienced >60% more naturally occurring chronic periodontal bone loss. In conclusion, MFG-E8 is a novel homeostatic regulator of osteoclasts that could be exploited therapeutically to treat periodontitis and perhaps other immunological disorders associated with inflammatory bone loss.
Figures
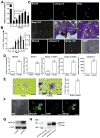


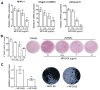
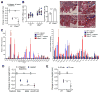
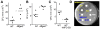
Similar articles
-
MFG-E8, a novel homeostatic regulator of osteoclastogenesis.Inflamm Cell Signal. 2014;1(5):e285. doi: 10.14800/ics.285. Inflamm Cell Signal. 2014. PMID: 26052544 Free PMC article.
-
Loss of milk fat globule-epidermal growth factor 8 (MFG-E8) in mice leads to low bone mass and accelerates ovariectomy-associated bone loss by increasing osteoclastogenesis.Bone. 2015 Jul;76:107-14. doi: 10.1016/j.bone.2015.04.003. Epub 2015 Apr 11. Bone. 2015. PMID: 25868798
-
Milk Fat Globule-Epidermal Growth Factor 8 (MFG-E8) Is a Novel Anti-inflammatory Factor in Rheumatoid Arthritis in Mice and Humans.J Bone Miner Res. 2016 Mar;31(3):596-605. doi: 10.1002/jbmr.2721. Epub 2015 Nov 9. J Bone Miner Res. 2016. PMID: 26391522 Free PMC article.
-
Role of milk fat globule-epidermal growth factor 8 in osteoimmunology.Bonekey Rep. 2016 Jul 20;5:820. doi: 10.1038/bonekey.2016.52. eCollection 2016. Bonekey Rep. 2016. PMID: 27579162 Free PMC article. Review.
-
Milk fat-globule epidermal growth factor 8: A potential Regulator of Cutaneous Wound Healing.Mol Biol Rep. 2022 Sep;49(9):8883-8893. doi: 10.1007/s11033-022-07365-6. Epub 2022 May 17. Mol Biol Rep. 2022. PMID: 35581508 Review.
Cited by
-
MFG-E8, a novel homeostatic regulator of osteoclastogenesis.Inflamm Cell Signal. 2014;1(5):e285. doi: 10.14800/ics.285. Inflamm Cell Signal. 2014. PMID: 26052544 Free PMC article.
-
DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates.Sci Transl Med. 2015 Sep 30;7(307):307ra155. doi: 10.1126/scitranslmed.aac5380. Sci Transl Med. 2015. PMID: 26424570 Free PMC article.
-
MFG-E8 Plays an Important Role in Attenuating Cerulein-Induced Acute Pancreatitis in Mice.Cells. 2021 Mar 25;10(4):728. doi: 10.3390/cells10040728. Cells. 2021. PMID: 33806041 Free PMC article.
-
Benzydamine inhibits osteoclast differentiation and bone resorption via down-regulation of interleukin-1 β expression.Acta Pharm Sin B. 2020 Mar;10(3):462-474. doi: 10.1016/j.apsb.2019.11.004. Epub 2019 Nov 8. Acta Pharm Sin B. 2020. PMID: 32140392 Free PMC article.
-
Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms.Prog Neurobiol. 2017 Nov;158:94-131. doi: 10.1016/j.pneurobio.2017.07.004. Epub 2017 Jul 23. Prog Neurobiol. 2017. PMID: 28743464 Free PMC article. Review.
References
-
- Aziz M, Jacob A, Matsuda A, Wang P. Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis. 2011;16:1077–1086. - PubMed
-
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. - PubMed
-
- Aziz MM, Ishihara S, Mishima Y, Oshima N, Moriyama I, Yuki T, Kadowaki Y, Rumi MA, Amano Y, Kinoshita Y. MFG-E8 attenuates intestinal inflammation in murine experimental colitis by modulating osteopontin-dependent alphavbeta3 integrin signaling. J Immunol. 2009;182:7222–7232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

