Evidence for the selective reporting of analyses and discrepancies in clinical trials: a systematic review of cohort studies of clinical trials
- PMID: 24959719
- PMCID: PMC4068996
- DOI: 10.1371/journal.pmed.1001666
Evidence for the selective reporting of analyses and discrepancies in clinical trials: a systematic review of cohort studies of clinical trials
Abstract
Background: Most publications about selective reporting in clinical trials have focussed on outcomes. However, selective reporting of analyses for a given outcome may also affect the validity of findings. If analyses are selected on the basis of the results, reporting bias may occur. The aims of this study were to review and summarise the evidence from empirical cohort studies that assessed discrepant or selective reporting of analyses in randomised controlled trials (RCTs).
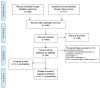
Methods and findings: A systematic review was conducted and included cohort studies that assessed any aspect of the reporting of analyses of RCTs by comparing different trial documents, e.g., protocol compared to trial report, or different sections within a trial publication. The Cochrane Methodology Register, Medline (Ovid), PsycInfo (Ovid), and PubMed were searched on 5 February 2014. Two authors independently selected studies, performed data extraction, and assessed the methodological quality of the eligible studies. Twenty-two studies (containing 3,140 RCTs) published between 2000 and 2013 were included. Twenty-two studies reported on discrepancies between information given in different sources. Discrepancies were found in statistical analyses (eight studies), composite outcomes (one study), the handling of missing data (three studies), unadjusted versus adjusted analyses (three studies), handling of continuous data (three studies), and subgroup analyses (12 studies). Discrepancy rates varied, ranging from 7% (3/42) to 88% (7/8) in statistical analyses, 46% (36/79) to 82% (23/28) in adjusted versus unadjusted analyses, and 61% (11/18) to 100% (25/25) in subgroup analyses. This review is limited in that none of the included studies investigated the evidence for bias resulting from selective reporting of analyses. It was not possible to combine studies to provide overall summary estimates, and so the results of studies are discussed narratively.
Conclusions: Discrepancies in analyses between publications and other study documentation were common, but reasons for these discrepancies were not discussed in the trial reports. To ensure transparency, protocols and statistical analysis plans need to be published, and investigators should adhere to these or explain discrepancies.
Conflict of interest statement
Three of the authors (KD, PRW and DGA) are authors on four of the included studies ,,,.
References
-
- Hutton JL, Williamson PR (2000) Bias in meta-analysis due to outcome variable selection within studies. Appl Stat 49: 359–370.
-
- Song F, Parekh S, Hooper L, Loke YK, Ryder J, et al. (2010) Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess 14: 1–193. - PubMed
-
- Page MJ, McKenzie JE, Forbes A (2013) Many scenarios exist for selective inclusion and reporting of results in randomized trials and systematic reviews. J Clin Epidemiol 66: 524–537. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources