A new role for myosin II in vesicle fission
- PMID: 24959909
- PMCID: PMC4069105
- DOI: 10.1371/journal.pone.0100757
A new role for myosin II in vesicle fission
Abstract
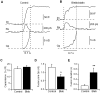
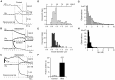
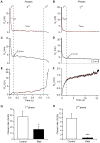
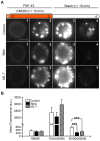
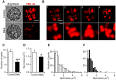
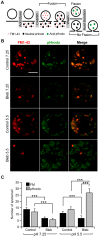
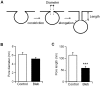
An endocytic vesicle is formed from a flat plasma membrane patch by a sequential process of invagination, bud formation and fission. The scission step requires the formation of a tubular membrane neck (the fission pore) that connects the endocytic vesicle with the plasma membrane. Progress in vesicle fission can be measured by the formation and closure of the fission pore. Live-cell imaging and sensitive biophysical measurements have provided various glimpses into the structure and behaviour of the fission pore. In the present study, the role of non-muscle myosin II (NM-2) in vesicle fission was tested by analyzing the kinetics of the fission pore with perforated-patch clamp capacitance measurements to detect single vesicle endocytosis with millisecond time resolution in peritoneal mast cells. Blebbistatin, a specific inhibitor of NM-2, dramatically increased the duration of the fission pore and also prevented closure during large endocytic events. Using the fluorescent markers FM1-43 and pHrodo Green dextran, we found that NM-2 inhibition greatly arrested vesicle fission in a late phase of the scission event when the pore reached a final diameter of ∼ 5 nm. Our results indicate that loss of the ATPase activity of myosin II drastically reduces the efficiency of membrane scission by making vesicle closure incomplete and suggest that NM-2 might be especially relevant in vesicle fission during compound endocytosis.
Conflict of interest statement
Figures


Similar articles
-
Mechanisms of granule membrane recapture following exocytosis in intact mast cells.J Biol Chem. 2013 Jul 12;288(28):20293-305. doi: 10.1074/jbc.M113.459065. Epub 2013 May 24. J Biol Chem. 2013. PMID: 23709219 Free PMC article.
-
Dynasore inhibits rapid endocytosis in bovine chromaffin cells.Am J Physiol Cell Physiol. 2009 Aug;297(2):C397-406. doi: 10.1152/ajpcell.00562.2008. Epub 2009 Jun 10. Am J Physiol Cell Physiol. 2009. PMID: 19515902
-
An acto-myosin II constricting ring initiates the fission of activity-dependent bulk endosomes in neurosecretory cells.J Neurosci. 2015 Jan 28;35(4):1380-9. doi: 10.1523/JNEUROSCI.3228-14.2015. J Neurosci. 2015. PMID: 25632116 Free PMC article.
-
Mechanisms of synaptic vesicle recycling illuminated by fluorescent dyes.J Neurochem. 1999 Dec;73(6):2227-39. doi: 10.1046/j.1471-4159.1999.0732227.x. J Neurochem. 1999. PMID: 10582580 Review.
-
Vesicle formation and endocytosis: function, machinery, mechanisms, and modeling.Antioxid Redox Signal. 2009 Jun;11(6):1301-12. doi: 10.1089/ars.2008.2397. Antioxid Redox Signal. 2009. PMID: 19113823 Free PMC article. Review.
Cited by
-
Myosin II activity is required for structural plasticity at the axon initial segment.Eur J Neurosci. 2017 Jul;46(2):1751-1757. doi: 10.1111/ejn.13597. Epub 2017 May 17. Eur J Neurosci. 2017. PMID: 28452088 Free PMC article.
-
ALPK1 phosphorylates myosin IIA modulating TNF-α trafficking in gout flares.Sci Rep. 2016 May 12;6:25740. doi: 10.1038/srep25740. Sci Rep. 2016. PMID: 27169898 Free PMC article.
-
Phosphatidylserine is critical for vesicle fission during clathrin-mediated endocytosis.J Neurochem. 2020 Jan;152(1):48-60. doi: 10.1111/jnc.14886. Epub 2019 Oct 27. J Neurochem. 2020. PMID: 31587282 Free PMC article.
-
Myosin light chain kinase facilitates endocytosis of synaptic vesicles at hippocampal boutons.J Neurochem. 2016 Jul;138(1):60-73. doi: 10.1111/jnc.13635. Epub 2016 May 16. J Neurochem. 2016. PMID: 27062289 Free PMC article.
-
Frontline Science: Tumor necrosis factor-α stimulation and priming of human neutrophil granule exocytosis.J Leukoc Biol. 2017 Jul;102(1):19-29. doi: 10.1189/jlb.3HI0716-293RR. Epub 2017 Jan 17. J Leukoc Biol. 2017. PMID: 28096297 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

